Term: Level of Evidence
The definition of level of evidence, according to the National Cancer Institute, is "a ranking system used to describe the strength of the results measured in a clinical trial or research study. The design of the study and the endpoints measured affect the strength of the evidence."
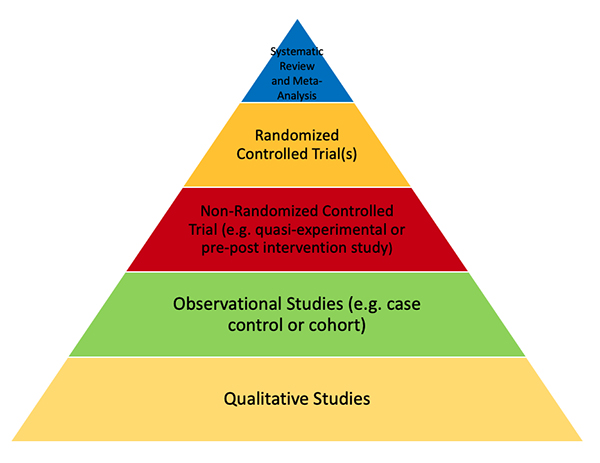
Studies that are higher quality will lead to a higher level of evidence. An example of a pyramid with Levels of Evidence is provided below. Note that Randomized Controlled Trials are the highest level of evidence in single trials. Meta-analyses of homogenous randomized controlled trials can also provide more information than small randomized controlled trials.
Lots of information can affect the quality of a study. Level of evidence is only one such factor that should be considered when assessing study quality. Various groups have published Level of Evidence Pyramids, and they have some minor differences, but generally follow the theme below. Some levels of evidence pyramids include expert opinion at the bottom below qualitative studies. Others leave out meta-analysis, as these studies can lead to false conclusions if not done well or when completed with poorly done initial trials.