A 31-year-old female G4P4004 presents via EMS after having delivered a 39-week baby boy at home. EMS states the baby was already delivered when they arrived; they clamped and cut the cord and transported the baby without issues. As you confirm the baby is healthy, the nurse states, “There is a lot of blood on the sheets.” Mom appears pale and anxious with HR 115 and BP 95/60. How do you approach this situation?
Introduction
For the purposes of this article, we will focus on the mother rather than the neonate. Postpartum hemorrhage (PPH) has been classically defined as blood loss > 500 mL and 1000 mL after vaginal and caesarean section, respectively. More recently, the American College of Obstetricians and Gynecologists redefined PPH as blood loss > 1000 mL or signs and symptoms of hypovolemia in the setting of bleeding within the first 24 hours.1 PPH is the leading cause of maternal mortality worldwide, and in the U.S. it accounts for about 15% of maternal mortality.2-4 While it is difficult to predict who will suffer from PPH, risk factors include history of PPH, known coagulopathy, prolonged labor, chorioamnionitis, multiple gestation, and > 4 previous deliveries (vaginal or caesarean). Recognition of patient risk can help emergency providers better prepare for PPH, ideally before deterioration in vital signs.
Etiology of PPH Normal physiologic changes in pregnancy include an increase in stroke volume, circulating blood volume, and procoagulant factors which prepare the maternal system for expected blood loss during delivery.2 However, beyond 20% blood loss, the systemic vascular resistance cannot compensate and the patient’s physiology behaves similar to that described in the Advanced Trauma Life Support classification of hemorrhage shock.2,5 The majority of PPH etiologies can be remembered by the 4T’s: Tone, Trauma, Tissue, and Thrombin.
Tone of the uterus (ie, atony) comprises 80% of PPH.1,2 At the time of delivery, blood flow to the uterus reaches 500-900 mL/min. After delivery, high levels of oxytocin help potentiate uterine contraction and vasoconstriction of uterine arteries in order to minimize blood loss.1 The pathophysiology of uterine atony is often attributed to risk factors:
- Impaired contractions from local inflammation and acidosis of uterine tissue (chorioamnionitis)
- Down regulation of oxytocin receptors (prolonged labor)
- Diminished actin-myosin interaction from an enlarged uterus (fetal macrosomia, multiple-gestation pregnancies).3
Importantly, atony can also occur in the absence of risk factors.
Trauma during delivery is the second most common cause of PPH. 80% of the lacerations sustained (most often to the vagina or perineum) are minor.3 If bleeding from a laceration does not stop with pressure, repair by a qualified provider is required. Packing can be placed to tamponade bleeding until appropriate repair can be performed. Cervical lacerations, often more difficult to visualize, should also be kept in the differential for continued postpartum bleeding of unknown source.
Tissue refers to retained products of conception (ie, placenta). The disruption of the placenta from the uterine wall helps stimulate uterine contraction. The placenta should always be examined to ensure it is fully intact. Bedside ultrasound showing a thickened endometrial stripe or a mass in the uterus could indicate retained products.1 Treatment involves manual removal of the retained products, which may need to occur in the operating room if analgesia cannot be achieved adequately for the exam.
Thrombin refers to both inherited and acquired coagulation disorders. Often, patients will present knowing their past medical history, which will allow you to provide therapies targeted to hemophilia A, B, or von Willebrand disease. An acquired coagulopathy can occur as a result of placental abruption or amniotic fluid embolism, leading to significant hemodynamic compromise from disseminated intravascular coagulation or hypofibrinogenemia.1 Bleeding related to coagulopathies involves replacing the factor deficiency.
Management of Uterine Atony
It is easy to be distracted by the neonate and lose precious time in the maternal resuscitation. Physicians should approach PPH just as they approach other resuscitations. Start with the ABCs, large bore IV access, and place the patient on a monitor (Figure 1).
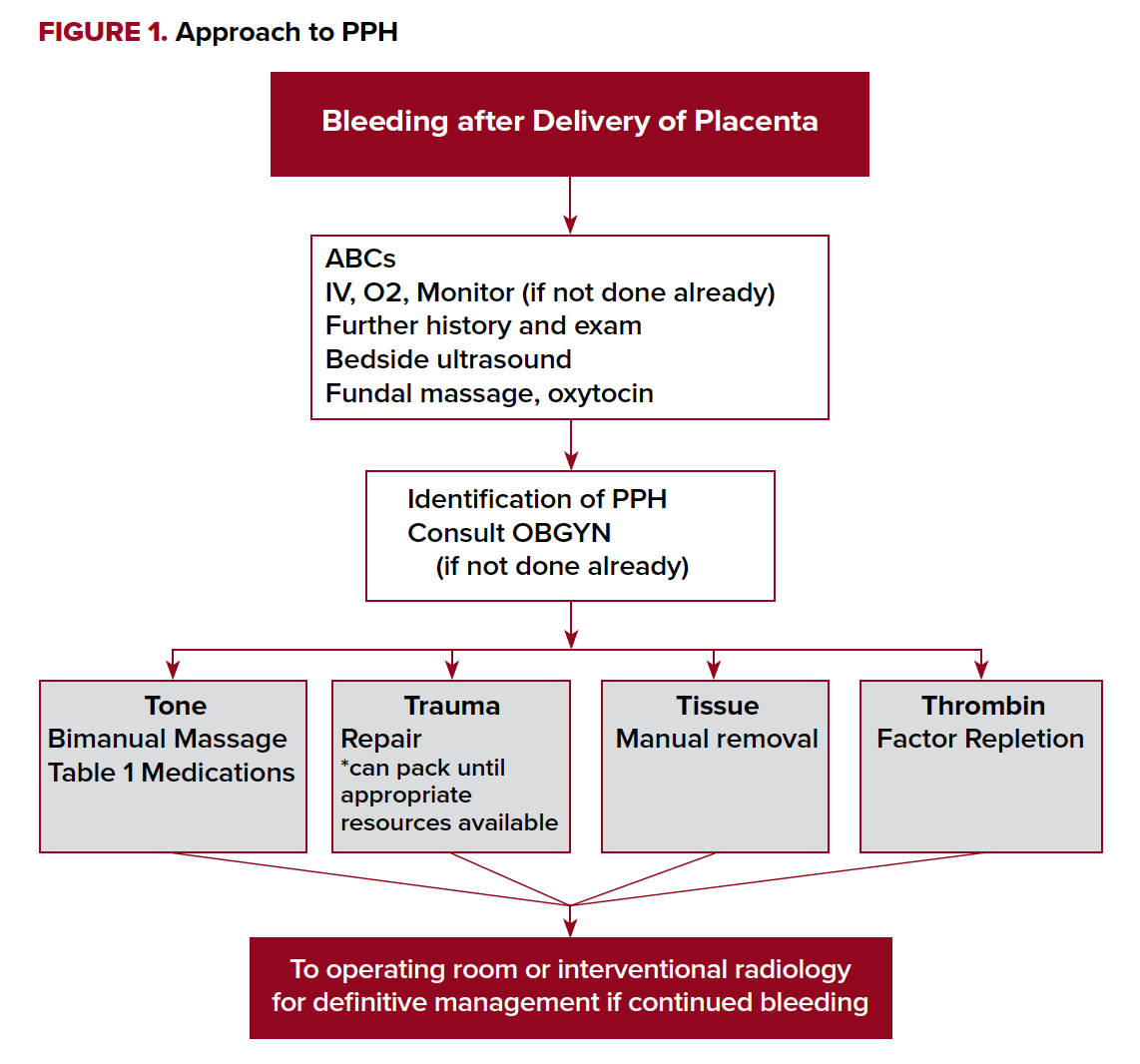
Providers should then target care towards the underlying cause. As discussed previously, thorough pelvic and placental exams are required to determine an etiology of the PPH. A soft, “boggy” uterus suggests bleeding is due to uterine atony. If atony is diagnosed, start with bimanual massage. This involves compressing the uterus between both hands, one internally and the other externally. Uterotonics can then be used to encourage the uterus to clamp down. Oxytocin, a hormone naturally produced by the body to stimulate contractions, can help with achieving uterine tone. For every birth, 20 units of oxytocin in 1 L normal saline should be given intravenously. If you do not have IV access, 10 units of oxytocin can be given intramuscularly to the buttock as an alternative. In the setting of PPH, an additional 20 units intravenously or 10 units intramuscularly can be given. A 2013 Cochrane review found oxytocin decreased bleeding by more than 500 mL regardless of the dose given.6
Additional medications include misoprostol, carboprost, and methylergonovine. The dosing of and contraindications to these medications are detailed in Table 1.
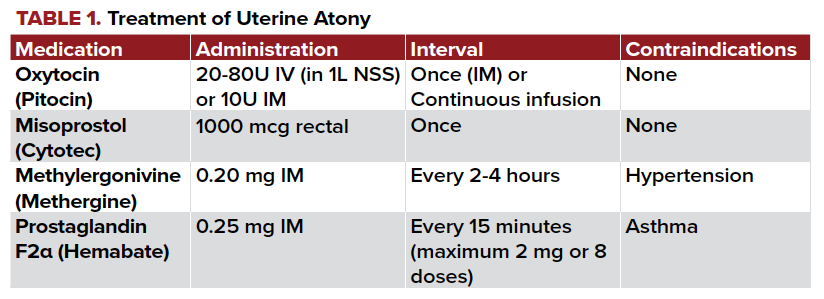
If uterotonics are not achieving their desired effect, the antifibrinolytic tranexamic acid (TXA) may be considered. TXA indirectly stabilizes clot formation by inhibiting plasminogen from working to break down fibrin. While studies have shown decreased blood loss with TXA, it is still an area of active research.1,3 Some clinicians suggest TXA to be used as an adjunct to other therapies in severe PPH or those who refuse blood transfusion.
More advanced therapies are required if uterotonics and uterine massage are unsuccessful. It is reasonable to activate your institution-specific massive transfusion protocol if there is persistent bleeding and concern for anticipated blood loss expected to lead to hemodynamic instability. At our hospital, for example, activation of massive transfusion automatically supplies 6 units of packed red blood cells, 1 unit platelets (pooled) and 6 units of plasma.
Mechanical approaches to stopping bleeding include uterine tamponade, most commonly performed with a Bakri balloon. If this is unavailable, multiple Foley catheters or uterine packing can be considered.
Bleeding refractory to these therapies requires invasive interventions. If the patient is deemed stable and interventional radiology is immediately available at your institution, embolization of the uterine arteries can prevent need for emergent hysterectomy in 85% of qualifying women. This procedure has minimal side effects and preserves fertility.1 When this fails or a patient is unstable, open surgery may be required to perform B-lynch sutures, hypogastric artery ligation, or, ultimately, a hysterectomy.
Precipitous deliveries are not an uncommon reason for patients to present to the emergency room. Postpartum hemorrhage cannot be consistently predicted and requires emergency providers to quickly recognize this complication and be prepared to initiate therapies unique to this patient population. Understanding what resources are immediately available in the emergency department, as well as resources they can call upon outside the department, can maximize outcomes for postpartum patients.
References
1. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstetrics Gynecol. 2017;130(4):e168-e186.
2. Hamm RF, Wang EY. Unique concepts of hemorrhagic shock and bleeding in the peripartum patient. In: Pascual JL, Cannon J, eds. Hemorrhagic Shock: Recognition, Pathophysiology and Management. Nova Science Publishers;2017.
3. Papazian J, Kacmar RM. Obstetric Hemorrhage: Prevention, Recognition, and Treatment. Adv Anesth. 2017;35(1):65-93.
4. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2(6):e323-333.
5. Cannon JW. Hemorrhagic Shock. N Engl J Med. 2018;378(4):370-379.
6. Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013(10):Cd001808.