Transcranial Doppler offers exciting potential - but in what settings? This literature review evaluates its use in trauma, ICU, pediatric, rural, and international settings.
Transcranial Doppler (TCD) ultrasound was first utilized in 1982 as a noninvasive way to evaluate cranial blood flow, particularly of the middle, anterior, and posterior cerebral arteries (MCA, ACA, and PCA, respectively).1 Since then, it has gained momentum as a tool to evaluate intracranial emergencies including post-aneurysmal hemorrhage, middle cerebral artery (MCA) vasospasm, cerebral ischemia/arterial occlusion, and midline shift.2,3 While TCD is at a disadvantage to CT in definitive diagnostic ability, it boasts distinct advantages that CT cannot replicate. Neurologic emergencies rely heavily on speed of provider recognition and appropriate escalation of care. TCD has the ability to provide dynamic, real-time information on intracranial processes in a way that a single snapshot in time, as obtained by CT, cannot. While it can be difficult to obtain a good acoustic window in 5-20% of patients,4 TCD allows us an opportunity to diagnose and treat intracranial pathologies when other modes of brain imaging are not available. A comparison of the advantages and disadvantages can be seen in Table 1.
Table 1. Advantages and Disadvantages of TCD in the ED
|
Advantages |
Disadvantages |
|
Safe, non-invasive, no radiation |
Highly user dependent |
|
Provides real-time information on status of cerebral arteries |
Not as accurate as current gold standard brain imaging |
|
Bedside technique that is easily repeatable |
Reduced acoustic view in 5-20% of patients4 |
|
Less expensive than CT |
Time intensive |
In addition to the aforementioned clinical uses, cranial ultrasound has been shown to have emerging roles in the evaluation of pediatric skull fracture and other cranial pathologies.5 In the scope of emergency medicine practice, four promising applications for TCD have been increasingly recognized in the literature for their potential to aid providers in the care of neurologic emergencies. Here, we will review the relevant anatomy in TCD, followed by a review of the literature on four promising applications of emergency TCD: rule-in vasospasm, MCA occlusion, midline shift, and pediatrics. While we will focus on these four aspects, there are many more indications for TCD as detailed in Table 2.
Table 2. Indications for Transcranial Doppler
|
Cerebral vasospasm (including post subarachnoid hemorrhage)6,7 |
|
CVA/Stroke8,9 |
|
Identification of midline shift10,11 |
|
Elevated intracranial pressure12 |
|
Evaluation of thrombolysis efficacy13 |
|
Determination of brain death14 |
|
Pediatric skull fracture15,16 |
|
Monitor cerebral perfusion pressure during resuscitation17,18 |
|
Syncope or positional vertigo19,20 |
I: TCD Basics
Understanding the current and future applications of TCD in the emergency department first requires a review of basic concepts. In this section, we will review the anatomy, probe locations, as well as basic concepts and definitions.
Anatomy
The Circle of Willis is of central importance in TCD (see Figure 1).3
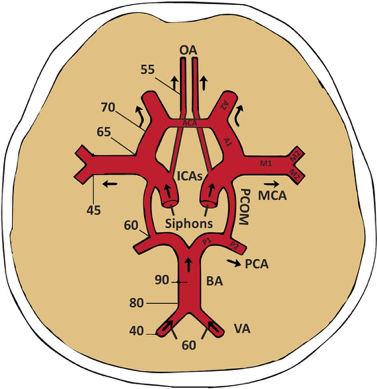
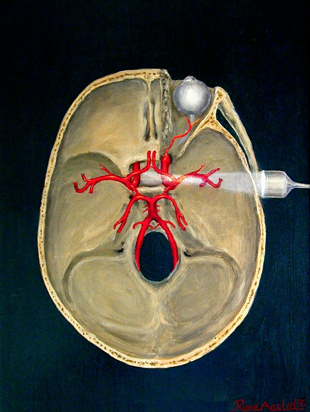
Figure 1. A) A review of the major intracranial arteries in the Circle of Willis. Also shown is flow direction of various arteries and depths of insonation (in mm) for an average human skull. OA: ophthalmic artery, ICA: internal carotid artery, MCA: middle cerebral artery, BA: basilar artery, VA: vertebral artery, PCA: posterior cerebral artery, PCOM: posterior communicating artery, ACA: anterior cerebral artery.3 B) View of Circle of Willis and right MCA from transtemporal positioning of 2Mhz probe.
Probe Placement
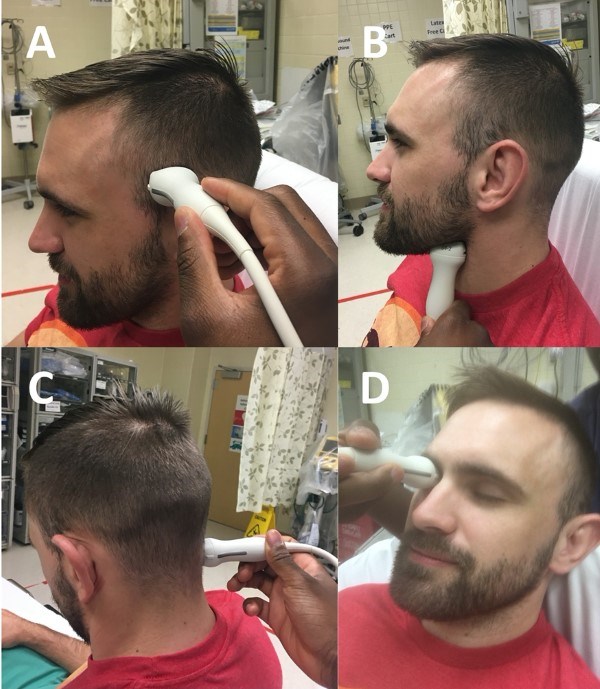
Now that we have reviewed the relevant anatomy, let’s review the transducer placement, which is key to obtaining a good acoustic window. There are four primary probe locations in TCD: transtemporal, suboccipital, orbital, and submandibular, as detailed in Table 3.
Table 3: Transducer windows and clinical application3
|
Window |
Location of Probe |
Clinical Application |
|
Transtemporal (most common) |
Ultrasound probe (with indicator pointed towards the patient’s face) placed over temporal area, slightly above zygomatic arch and immediately in front of tragus of the ear (Figure 2A) |
Evaluation of MCA measurements useful in observing cerebral vasospasm and ischemic stroke |
|
Suboccipital |
Side bend and rotate head to one direction and place probe just below and medial to mastoid process, directing transducer slightly medially towards bridge of the nose or contralateral eye (Figure 2C) |
Determination of flow signals from ipsilateral vertebral artery |
|
Submandibular |
Probe placed laterally under the jaw, anteromedially to SCM with indicator pointing slightly upwards and medial (Figure 2B) |
Evaluation of distal ICA |
|
Transorbital |
Probe gently placed over closed eyelid and aimed slightly superomedially (Figure 2D) |
Evaluation of ipsilateral ophthalmic artery and ICA siphon |
Figure 2. Ultrasound Transducer Windows
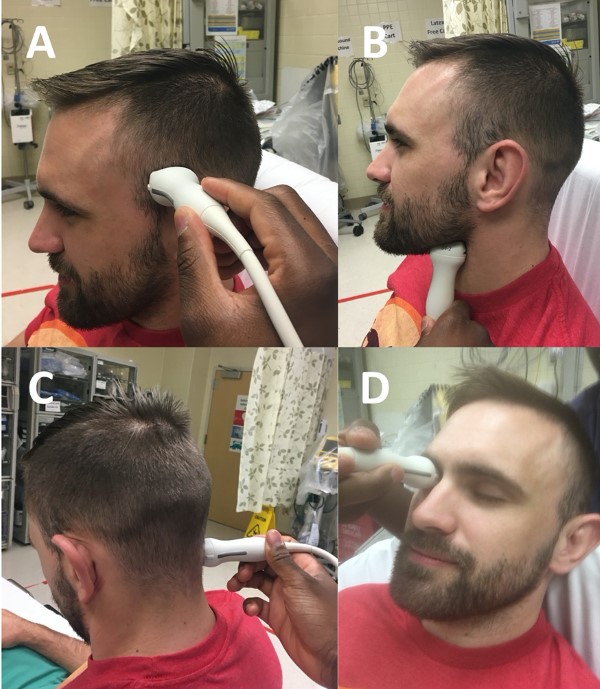
Figure 2. Four sonographic TCD windows depicted using 2MHz ultrasound probe. A) transtemporal window B) submandibular window C) suboccipital window D) transorbital window3
Terminology
Table 4 defines common terminology and formulas used in evaluation of TCD3
|
Term |
Definition |
Clinical Application |
|
Peak Systolic Velocity (PSV in cm/s) |
Initial peak on TCD waveform during each cardiac cycle |
Rapid upstroke indicates lack of severe stenotic or occlusive lesion between observed cerebral artery and heart |
|
End Diastolic Velocity (EDV in cm/s) |
Should be between 20% and 50% of PSV value |
Indicates low resistance intracranial arterial flow, a normal finding |
|
Mean Flow Velocity (MFV in cm/s) |
MFV = EDV + .33 (PSV – EDV) |
MCA should have highest MFV in all cerebral arteries observed |
|
Pulsatility index (PI) |
PI = (PSV-EDV)/MFV |
Used to measure resistance to intra-arterial flow. Value >1.2 represents high resistance to flow |
|
Resistance Index (RI) |
RI = (PSV-EDV)/PSV |
Another measurement of flow resistance, this time distal to the area insonated. Normal <0.75 |
|
Lindegaard Ratio (LR) |
LR = ipsilateral MCA MFV/ipsilateral |
Ratio used to detect cerebral vasospasm. Ratios of 3-6 indicate mild to moderate vasospasm. >6 = severe vasospasm21 |
Figure 3. TCD Velocities and Measurements
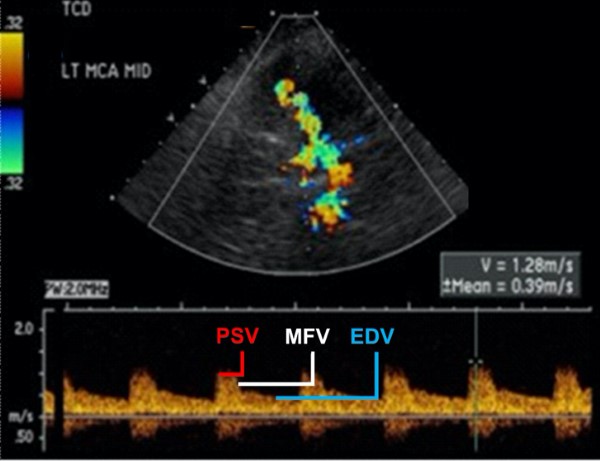
Figure 3. Common measurements taken in TCD evaluation using transtemporal window [3]: PSV (peak systolic velocity in cm/s). EDV(end diastolic velocity in cm/s). Note how EDV is 38% (25.3/65.9) of PSV. MFV (mean flow velocity in cm/s). Note pulsatility index (PI) of 1.05 and depth and angle of insonation (3.5cm).
II: Rule-in Vasospasm
Cerebral vasospasm is a common phenomenon following aneurysmal subarachnoid hemorrhage that can lead to worsened and irreversible cerebral ischemia, with early detection proving critical to improving patient outcomes.22 Utilizing the temporal view described above, TCD and Transcranial Color Doppler (TCCD) sonography have proven to be useful as a screening tool in identifying middle cerebral artery (MCA) vasospasm.3,23 Evidence for the usefulness of TCD/TCCD in detecting vasospasm in other cerebral arteries is lacking at this time. Vasospasm is identified by measuring an abnormally high mean flow velocity (MFV) in the affected vessel while surrounding vasculature remains in the low to normal velocity range.2 Per a meta-analysis authored by Mastantuono et al.,24 TCD and TCCD detection of MCA vasospasm had a pooled sensitivity of 66.7% and 81.5% with a pooled specificity of 89.5% and 96.6%, respectively, when compared to the “gold standard” of angiography. A meta-analysis by Lysakowski et al. showed a pooled sensitivity and specificity of 67% and 99%, respectively.25 Given these data, TCD/TCCD has been validated as a useful screening tool to rule-in MCA vasospasm, not to rule it out. The advantages of this technique include lack of radiation and contrast agent, as well as real-time evaluation of intracranial flow dynamics.However, the use of TCD/TCCD relies heavily on operator experience and skill-level due to the complexity and tortuous nature of cerebral vasculature as well as the numerous factors that affect cerebral blood flow.3,23 The primary factors affecting cerebral blood flow are age, gender, hematocrit, viscosity, carbon dioxide, temperature, blood pressure, and mental or motor activity.23
Of note, cerebral vasospasm typically occurs 3-14 days after SAH,24 so it may be difficult to use TCD for vasospasm monitoring in the emergency setting. That being said, progressive increases in MFV early in the setting of SAH were shown to be predictive of vasospasm.24
III: Change in Midline Shift
Midline shift (MLS) following a traumatic brain injury or cerebrovascular incident can lead to rapid deterioration of the patient’s condition and is indicative of poor prognosis.26 TCD may be used to identify this midline shift, especially in cases when immediate CT may not be an appropriate or available option. According to Montrief et al.3 and Blanco et al., MLS can be most reliably calculated through the operator’s visualization of the transtemporal window to measure the distance from each temporal bone to the midline of the third ventricle (see Figure 4 below). Additionally, the operator should measure the distance between the temporal bones to ensure that it is equal to the sum of the 2 temporal bone-to-midline measurements; this checks the reliability of the measured distances.
The following equation is then utilized to calculate the midline shift:
Midline shift = (distance A - distance B)/2
Note: A=distance from ipsilateral temporal bone to third ventricle midline, B=distance from contralateral temporal bone to third ventricle midline
If the calculated MLS is positive, then MLS is away from the ipsilateral side toward the contralateral side, with the inverse being true in the case of a negative MLS. TCD identification of significant MLS using this calculation has been shown to have a high degree of repeatability and reasonable correlation with CT findings (r2 ranged from 0.58 – 0.87).3,27,28
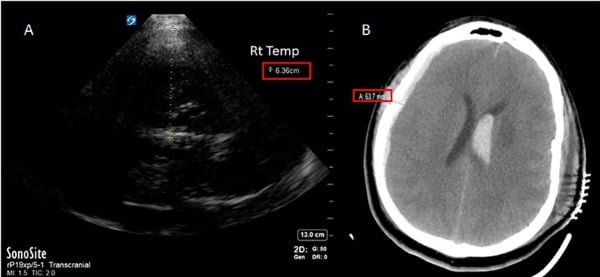 Figure 4. TCD used to identify midline shift. A) insonation from right temporal bone (via transtemporal window) to third ventricle shows distance of 6.36 cm. B) Follow-up CT scan confirming shift observed with TCD.3
Figure 4. TCD used to identify midline shift. A) insonation from right temporal bone (via transtemporal window) to third ventricle shows distance of 6.36 cm. B) Follow-up CT scan confirming shift observed with TCD.3
If a CT scan is not immediately available, using TCD to identify patients with deteriorating neurological status and concerns for mass effect could save valuable minutes, especially in locations that would need to transfer the patient to a tertiary care center for definitive treatment.
IV: MCA Occlusion
TCD can be utilized to assess for occlusion in cerebral arteries resulting in acute ischemic stroke, with the best evidence for use in the case of MCA occlusion. Signs of occlusion include decreased or absent arterial signal using doppler and sonographic evidence of collateral flow. To check for signs of primary stenosis, look for increased MFV at the site of luminal narrowing. Secondary stenosis can be observed as abnormal flow or decreased flow velocity distal to the lesion or increased pulsatility proximal to the lesion.29 MCA occlusion identification with TCD has reported sensitivities and specificities of, 93-100% and 92-98%, respectively.30,31,32 A limited number of studies have additionally shown utility in ICA occlusions.3,30
TCD is also a useful tool in monitoring MCA occlusions post thrombolytic therapy. TCD has a high positive predictive value (91%) for determining complete reperfusion of the MCA via comparative Doppler pulse wave velocity measurements which utilize the non-occluded contralateral artery flow rate as a standard. Full restoration of flow rates in the MCA without any distal signals of continued occlusion correlated well with TIMI grade III flow on CT angiography (complete reperfusion).33 Partial or no restoration of flow rates via TCD can be a good clinical indication for additional angiography and can aid in decision making for local thrombolytic therapy such as intra-arterial thrombolysis or mechanical thrombectomy.2,3,9,33
There is some evidence that TCD monitoring post thrombolytic therapy may act synergistically with tissue plasminogen activator (tPA)to achieve an ultrasound-enhanced thrombolytic effect.34 Through inducing mechanical pressure waves, ultrasound is believed to increase the surface area of thrombus exposed to intra-arterial tPA.34 When tPA was combined with TCD insonation in treatment of CVA, recanalization rates were higher (37.2%) than those treated with tPA alone (17.2%).34 However, recent publications have been inconsistent in demonstrating if there are true improvements in 90-day mortality and morbidity associated with TCD used adjunctively with intravenous thrombolytics.13,35 More research is certainly indicated before this treatment modality becomes a standard of care.
V: Pediatrics
Cranial ultrasound is used frequently in pediatric practice to assess infants and neonates at risk for intracranial pathology. The use of cranial ultrasound in this population takes advantage of the relatively large acoustic windows formed by open fontanelles, while reducing exposure to ionizing radiation and need for sedation during imaging. Traditionally, this imaging is conducted by ultrasound technicians and the studies are interpreted by radiologists.36
Recent cases and studies have shown an emerging role for cranial ultrasound in evaluating for pediatric skull fracture (PSFx), traumatic intracranial hemorrhage, and intraventricular hemorrhage using point-of-care ultrasound (POCUS) performed by emergency physicians. PSFx may be the most promising area of application for transcranial POCUS. While clinical decision tools such as PECARN have helped to reduce unnecessary imaging in children,37 their comparative effectiveness has been questioned.38 The addition of POCUS evaluation may augment the use of clinical decision tools, especially in situations with diagnostic uncertainty. Multiple studies report that POCUS in the ED for PSFx in children has sensitivities and specificities of 82-100% and 85.2-95%, respectively.39,40,16 In addition to PSFx, a pediatric ED case study indicates the potential utility of POCUS in traumatic intracranial hemorrhage.5 Additionally, cranial ultrasound is currently a popular modality used for newborn perinatal hemorrhage screening in the NICU setting.41 As with all POCUS studies, there is inherent variability due to physician experience and training levels.
Table 5. Summary: 4 TCD indications discussed, their technique, and clinical applications
|
Indication |
Technique |
Clinical Application |
|
Rule-in Vasospasm |
- Measure MFV (>200 cm/s is severe; <80cm/s is normal)29 |
- Serial measurements to monitor for post-SAH vasospasm - screen for post-SAH vasospasm |
|
Midline shift |
- Measure distance from temporal bone to third ventricle on ipsilateral and contralateral side and divide by 2. - If difference is positive, MLS is towards the ipsilateral side. - If difference is negative, MLS is towards the contralateral side. |
- serial monitoring in patients with subdural or other form of chronic intracranial bleed - reliable measurement of worsening neurologic status and useful when CT is not immediately available (patient unstable, repairs,CT in use, rural or international medicine). |
|
MCA occlusion |
- measure signal coming from MCA using doppler ultrasound. Similar to DVT or arterial occlusions, decreased or absent signal could indicate occlusion. |
- primarily useful for rapid identification of MCA occlusion when CT is unavailable. - potential use for ultrasound enhanced thrombectomy to improve recanalization |
|
Pediatrics |
- multiple techniques depending on specific indication |
- evaluation of skull fracture, fetal anemia, intracranial and intraventricular hemorrhage, and ischemic stroke |
Conclusion
Whenever we encounter a new technology or diagnostic approach, we must weigh the benefits versus the costs. Due to the repeatability of TCD, many of these applications are useful in the ICU environment where serial monitoring is vital. TCD can be reliably used for pediatric patients with skull fractures, and increased evidence is pointing towards usage for pediatric ischemic and hemorrhagic strokes. At this point, the data indicates that TCD has a high degree of utility in ICUs, pediatric, rural, and international EDs. However, for many of us who practice in trauma centers with CT consistently and readily available, the evidence currently recommends continued usage of CT/MRI for intracranial pathologies in the acute setting.
TCD is indeed an exciting field with a lot of potential and opportunities. As emergency physicians, we are trained to excel in providing timely, cost-effective treatment, and TCD has the potential to be widely beneficial. Regardless of the possibilities for this mode of diagnosis, it is imperative that we as an emergency medicine field decrease the variation in user ability. If we focus on increasing the level of experience and education for our medical students and physicians regarding POCUS, TCD is just one of many examples of the incredible potential that we could reach.
References
- Aaslid, R., et al. (1982). Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. Journal of Neurosurgery 57:769-774
- Blanco, P., et al. (2017). Applications of Transcranial Color-Coded Sonography in the Emergency Department. Journal of Ultrasound in Medicine, 36(6): 1251-1266
- Montrief, T., et al. (2019). Incorporation of Transcranial Doppler into the emergency department for the neurocritical care patient. American Journal of Emergency Medicine 37(6): 1144-1152
- M. Marinoni et al. Technical limits in transcranial Doppler recording: inadequate acoustic windows. Ultrasound Med Biol, 23 (8) (1997), pp. 1275-1277
- McCormick, T., et al. (2017). Point-of-care ultrasound for the detection of traumatic intracranial hemorrhage in infants: a pilot study. Pediatric emergency care 33.1: 18-20.
- J. Djelilovic-Vranic et al. Follow-up of vasospasm by transcranial Doppler sonography (TCD) in subarachnoid hemorrhage (SAH). Acta Inform Med, 25 (1) (2017), pp. 14-18.
- A. Rigamonti, A. Ackery, A.J. Baker. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth, 55 (2) (2008), pp. 112-123
- E.B. Ringelstein et al. Consensus on microembolus detection by TCD. International Consensus Group on Microembolus Detection. Stroke, 29 (3) (1998), pp. 725-729
- Sarkar S et al. Role of transcranial Doppler ultrasonography in stroke. Postgrad Med J. 2007;83(985):683‐689. doi:10.1136/pgmj.2007.058602
- A. Cattalani. Transcranial color-coded duplex sonography for evaluation of midline-shift after chronic-subdural hematoma evacuation (TEMASE): a prospective study. Clin Neurol Neurosurg, 162 (2017), pp. 101-107
- J.A. Llompart Pou et al. Monitoring midline shift by transcranial color-coded sonography in traumatic brain injury. A comparison with cranial computerized tomography. Intensive Care Med, 30 (8) (2004), pp. 1672-1675
- Cardim D, Robba C, Bohdanowicz M, et al. Non-invasive Monitoring of Intracranial Pressure Using Transcranial Doppler Ultrasonography: Is It Possible?. Neurocrit Care. 2016;25(3):473‐491. doi:10.1007/s12028-016-0258-6
- Alexandrov, A. V., et al. (2019) Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. The Lancet Neurology, 18(4), 338-347.
- Y. Li et al. Use of transcranial Doppler ultrasound for diagnosis of brain death in patients with severe cerebral injury. Med Sci Monit, 22 (2016), pp. 1910-1915
- Parri, N., et al. (2018). Point-of-care ultrasound for the diagnosis of skull fractures in children younger than two years of age. The Journal of pediatrics, 196, 230-236.
- Riera, A., & Chen, L. (2012). Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatric emergency care, 28(5), 420-425.
- J. Blumenstein et al. Cerebral flow pattern monitoring by transcranial Doppler during cardiopulmonary resuscitation. Anaesth Intensive Care, 38 (2) (2010), pp. 376-380
- B. Iordanova, L. Li, R.S.B. Clark, M.D. Manole. Alterations in cerebral blood flow after resuscitation from cardiac arrest. Front Pediatr, 5 (2017), p. 174
- Diehl RR, Linden D, Chalkiadaki A, Ringelstein EB, Berlit P. Transcranial Doppler during neurocardiogenic syncope. Clin Auton Res. 1996;6(2):71‐74. doi:10.1007/BF02291226
- Njemanze PC. Transcranial Doppler evaluation of syncope: an application in aerospace physiology. Aviat Space Environ Med. 1991;62(6):569‐572.
- Kirch, Jonathan et al. Advances in transcranial doppler US: Imaging Ahead. Radiographics. 33 (1).
- Nassar, S., et al. (2019). Vasospasm following aneurysmal subarachnoid hemorrhage: prediction, detection, and intervention. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 55(1)
- Purkayastha, S., et al. (2014). Transcranial Doppler Ultrasound: Technique and Application. Semin Neurol 32(4): 411-420
- Mastantuono, J., et al. (2018). Transcranial Doppler in the Diagnosis of Cerebral Vasospasm: An Updated Meta-Analysis. Critical Care Medicine, 46(10): 1665-1672
- Lysakowski, C., et al. (2001). Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: A systematic review. Stroke 32(10): 2292-2298
- Quattrocchi, K.B., et al. (1991). Quantification of midline shift as a predictor of poor outcome following head injury. Surg Neurol 35(3): 183-188
- Motuel, J., Biette, I., Srairi, M. et al. Assessment of brain midline shift using sonography in neurosurgical ICU patients. Crit Care 18, 676 (2014). https://doi.org/10.1186/s13054-014-0676-9
- Seidel G, Gerriets T, Kaps M, Missler U. Dislocation of the third ventricle due to space-occupying stroke evaluated by transcranial duplex sonography. J Neuroimag- ing 1996;6(4):227–30.
- N. de Riva et al. Transcranial Doppler pulsatility index: what it is and what it isn't. Neurocrit Care, 17 (1) (2012), pp. 58-66
- Demchuk, A., et al. (2003). Accuracy and Criteria for localizing arterial occlusion with transcranial doppler. Journal of Neuroimaging, 13: 17-27
- Camerlingo, M., et al. (1993) Transcranial Doppler in acute ischemic stroke of the middle cerebral artery territories. Acta neurologica scandinavica, 88(2), 108-111.
- Baumgartner, R. W., et al. (1999) Assessment of≥ 50% and< 50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke, 30(1), 87-92.
- Burgin, S., et al. (2011). Transcranial Doppler Ultrasound Criteria for Recanalization After Thrombolysis for Middle Cerebral Artery after Stroke. Stroke 31(5): 1128-1132
- Tsivgoulis, et al. (2010). Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke 41.2: 280-287
- Mazya, M. V., et al. & SITS Investigators. (2018) Impact of transcranial doppler ultrasound on logistics and outcomes in stroke thrombolysis: results from the SITS-ISTR. Stroke, 49(7), 1695-1700.
- Orman, G., et. al. (2015). Neonatal head ultrasonography today: a powerful imaging tool! Journal of Neuroimaging, 25(1), 31-55.
- Ballard, Dustin W. et al. (2019). Implementation of a Clinical Decision Support System for Children With Minor Blunt Head Trauma Who Are at Nonnegligible Risk for Traumatic Brain Injuries. Annals of Emergency Medicine, Volume 73, Issue 5, 440–451
- Babl, Franz E. et al. (2018). Accuracy of Clinician Practice Compared With Three Head Injury Decision Rules in Children: A Prospective Cohort Study. Annals of Emergency Medicine, Volume 71, Issue 6, 703-710
- Parri, N., et al. (2018). Point-of-care ultrasound for the diagnosis of skull fractures in children younger than two years of age. The Journal of pediatrics, 196, 230-236.
- Parri, N., et al. (2013). Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. The Journal of emergency medicine, 44(1), 135-141.
- Gupta, S. N., et al. (2009) Intracranial hemorrhage in term newborns: management and outcomes. Pediatric neurology, 40(1), 1-12.