A 36-year-old woman presented to the ED at a large national referral hospital in Uganda. She was referred there from a regional hospital, where she had been diagnosed with refractory heart failure. Despite being treated with diuretics as well as empiric thiamine for possible beriberi, her symptoms had progressively worsened.
The patient had a 1-year history of worsening neck swelling, a 2-week history of bilateral lower extremity edema, and difficulty breathing. Associated symptoms included palpitations, easy fatigability, orthopnea, dry cough, unintentional weight loss, excessive sweating, fever, and heat intolerance. Other symptoms included headache, dizziness, blurring of vision, and diplopia.
A review of her prior medical history revealed that the patient was treated for pulmonary tuberculosis (PTB) 3 years prior but did not finish the course of antibiotics. Repeat sputum testing for TB was negative. She had no other known chronic illness or medication, reported regular alcohol use with a CAGE score of 3, denied tobacco abuse, and worked as a produce vendor.
On examination, the patient was noted to be alert, had obvious proptosis, was tremulous, and exhibited severe muscular wasting. An anterior neck mass was noted, which measured 5x5 cm. This mass was noted to be firm, non-tender, mobile with swallowing, and had no retrosternal extension. Pemberton’s sign (in which the patient raises their arms to assess for facial plethora that would suggest venous obstruction) was negative.1
Vital signs were notable for a respiratory rate of 24 breaths/min, SpO2 89% RA, BP 131/73 mmHg, HR 125 beats/minute, and temperature 39.6 degrees Celsius. On chest auscultation, the patient had soft, low-pitched breath sounds with bilateral inframammary crackles. Heart sounds were notable for regular rate and rhythm with a pan systolic apical murmur radiating to the axilla but no rubs or gallops. She appeared clinically dehydrated, had mild pallor, grade II edema of the bilateral lower extremities, increased JVP, and positive hepatojugular reflux. She had no focal neurological deficits.
Prioritizing resuscitation, including airway, breathing, circulation, disability, and exposure, supplemental oxygen was administered for hypoxia at 15 L/minute, and fever was treated with intravenous (IV) paracetamol. Laboratory workup (Table 1) was notable for normal anion gap metabolic acidosis, acute kidney injury, hyperbilirubinemia, transaminitis, mild leukocytosis and anemia, and leukocyturia.
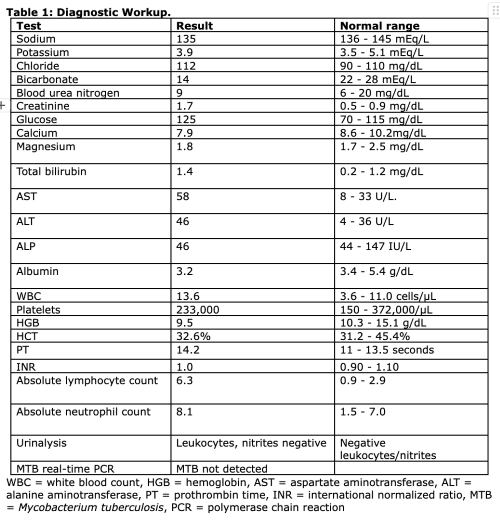
Given concern for thyroid storm, the patient was started on carbimazole 10 mg per os (PO) every 8 hours, propylthiouracil (PTU) 300 mg PO every 6 hours, dexamethasone 6 mg IV every 12 hours followed by prednisolone 15 mg every 24 hours, and propranolol 40 mg PO every 12 hours. Lugol’s iodine 2 drops PO was started 1 hour after the PTU and continued every 8 hours. Due to ongoing hypertension despite the propranolol, losartan 50 mg PO once daily was also initiated.
Point-of-care echocardiogram (ECHO) in the ED revealed LV hypertrophy with reduced ejection fraction, an incompressible inferior vena cava (IVC), and B-lines suggestive of acute pulmonary edema. An electrocardiogram (ECG) was obtained after the initiation of empiric therapy for thyroid storm and revealed sinus tachycardia. Chest X-ray was notable for small bilateral pleural effusions with cardiomegaly. Thyroid function tests (Table 2) resulted after the initiation of empiric antithyroid therapy while the patient was still in the ED and confirmed the suspected diagnosis of hyperthyroidism.
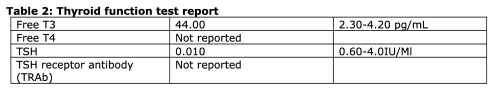
On day 2 of admission, the most likely diagnosis was felt to be dilated cardiomyopathy and congestive heart failure secondary to thyroid storm. Although TSH receptor antibody (TRAb) was not available for confirmation of Graves’ disease, this was presumed to be the underlying cause of the thyrotoxicosis, given that it is the most likely cause of hyperthyroidism in young patients. Cardiology and endocrinology were consulted, and the patient was admitted to the endocrine ward.
Discussion
Thyroid storm is a life-threatening spectrum of hyperthyroidism often caused by either poorly treated/undiagnosed hyperthyroidism or excessive use of thyroid hormone replacement therapy.2 It is a rare medical emergency with an estimated prevalence of 16% in patients hospitalized with thyrotoxicosis.2
Although the exact mechanism of thyroid storm is unclear, theories such as a rapid increase in thyroid hormone levels, sudden cessation of antithyroid drug therapy, increased sensitivity to catecholamines, and heightened responses to thyroid hormone at the cellular level have been documented.3
Though frequently preceded by an acute precipitating event such as infection or thyroid surgery, more than 25% of cases of thyroid storm may not have any identifiable precipitating factor.4
In the ED, thyroid storm is often seen in patients with undiagnosed or untreated hyperthyroidism,4 a similar presentation in our patient. Generally, Graves’ disease is the most common cause of thyroid storm (as with thyrotoxicosis); other causes include toxic multinodular goiter and toxic adenoma.5
While the presentation is often similar to that of thyrotoxicosis, thyroid storm can present with cardiopulmonary manifestations that can be life-threatening6 with reduced exercise tolerance being the most common cardiovascular manifestation. Patients with goiter may demonstrate a positive Pemberton’s sign in which bilateral arm elevation causes facial plethora and engorged neck veins due to obstruction of the thoracic inlet by the enlarged thyroid. Although less common, atrial fibrillation (AF), pulmonary hypertension, angina pectoris, and reversible heart failure with reduced ejection fraction occur in about 1-6% of patients with undiagnosed hyperthyroidism.7
This case underscores that thyrotoxicosis and/or thyroid storm can induce heart failure in patients without pre-existing heart conditions, a phenomenon that has been documented as thyrotoxic cardiomyopathy (TCM).8
TCM occurs in 1-2% of subjects with untreated/poorly controlled hyperthyroidism commonly associated with Graves' disease.2 A longstanding hyperdynamic state demonstrated by volume expansion, increased resting heart rate, enhanced LV contractility, and reduced systemic vascular resistance collectively result in an excessive increase in cardiac output before the eventual development of high-output heart failure.9 AF (if it occurs) further impairs the systolic dysfunction in thyrotoxicosis,10 resulting in the compromise of LV filling and, hence, diminished cardiac output. Combined with respiratory muscle weakness associated with thyrotoxicosis, this leads to cardiopulmonary failure, as seen in this patient. Both inspiratory and expiratory muscles are affected by thyrotoxicosis, often causing type 2 respiratory failure, which rapidly improves with antithyroid therapy.11,12
It should be noted that thiamine deficiency (i.e., beriberi) can also cause high-output heart failure and can present with similar symptoms to TCM.13 Though thiamine deficiency is not uncommon in low- and middle-income countries,14 and alcohol use disorder places patients at higher risk for this condition,13 the patient in this case did not improve with the administration of empiric thiamine. In addition, she had additional clinical examination findings (such as proptosis) and diagnostic findings (such as low TSH and elevated T3/T4), which pointed to TCM as the underlying etiology of her symptoms.
Management
Unlike many causes of heart failure often requiring diuresis, TCM responds well to rate control with reversal of hyperthyroidism. Cardiac remodeling seen in TCM is also frequently reversible, as evidenced by the restoration of normal LV function after attaining euthyroidism in patients who initially developed a reduced ejection fraction of <50%.15 Control of the sympathomimetic symptoms and inhibition of synthesis and release of thyroid hormones prevents decompensation.16,17 This was the case for our patient, who initially had a reduced EF on bedside ECHO, which normalized upon becoming euthyroid on follow-up.
Because of the life-threatening nature of this condition, early diagnosis and treatment are essential. In the ED, certain scoring criteria — such as Burch-Wartofsky Point Scale (BWPS) — have been suggested,18 based on the degree of organ dysfunction in the setting of thyrotoxicosis. In this scoring system, a score of 45 points or more is highly suggestive of thyroid storm, whereas a score below 25 makes thyroid storm unlikely, with a score of 25-44 suggestive of an impending storm.18 Although the BWPS has been documented to have a low specificity,19 our patient scored 65.
In combination with physical manifestations of thyroid disease, this was highly suggestive of thyroid storm, which informed the decision to initiate treatment of storm-awaiting low TSH levels and the abnormally high free T3/T4 levels that were later demonstrated in this case. Although we were unable to obtain laboratory markers for Graves’ disease, examination findings in support included diffuse goiter and orbitopathy.20 Other clues for Graves’ disease in this case included risk factors such as the female sex and age between 30-50 years.21
The mainstay of therapy for this patient involves beta-blockers, glucocorticoids, thionamides, and iodine.17,22 Thionamide therapy involves the use of antithyroid drugs such as propylthiouracil and methimazole to inhibit thyroid peroxidase (TPO), the chief enzyme responsible for the formation of T3 and T4.10 In addition to the antithyroid effects, early initiation of thionamides has also been shown to improve or even reverse heart failure.23
Likewise, beta-blocker therapy offers similar cardiac benefits and can reduce or even completely resolve cardiomyopathy, particularly when prolonged tachycardia is likely the cause, as seen in tachycardia-mediated cardiomyopathy.4 Evidence also suggests that PTU and propranolol prevent the conversion of T4 to T3, contributing to the reversal of thyrotoxic cardiomyopathy.24 The left ventricular ejection fraction (LVEF) subsequently may increase by 28% to 55% after treatment for thyrotoxicosis.17 Propranolol with beta-2 blockade increases systemic vascular resistance, abating cardiovascular collapse and potentially preventing AF (the most common cardiac arrhythmia seen in hyperthyroidism, occurring in up to 15% of patients).25
Glucocorticoids such as hydrocortisone and dexamethasone decrease the peripheral conversion of T4 to T3, prevent relative adrenal insufficiency due to hyperthyroidism, and help improve vasomotor symptoms.
Similarly, iodine compounds (ie, Lugol’s iodine or potassium iodide), administered at least 1 hour after the thionamide, block the release of preformed thyroid hormone, a paradox known as the Wolff-Chaikoff effect (which lasts for up to 2 weeks).
Administration of iodine compounds less than 1 hour after the thionamide can cause paradoxical worsening of symptoms by increasing thyroid hormone production.26
One novel component to be considered for the management of refractory hypertension despite beta-blockade in patients with thyroid storm is the addition of an angiotensin 2 receptor blocker (ARB), which was administered in this case. Thyroid hormones are suggested to upregulate angiotensin 2 receptors in the cardiac conduction tissue, which causes remodeling of the myocardium in cases of dilated cardiomyopathy. Literature suggests that ARBs may help reduce this remodeling, thus combatting one of the underlying causes of heart failure in thyroid storm.27
Finally, the definitive treatment for thyroid storm is the removal of the underlying cause, which likely represents surgery or radioactive iodine ablation therapy for a patient with Graves’ disease.
Case Resolution
The patient showed remarkable improvement during admission while receiving ongoing thionamide, steroid, iodine, and propranolol therapy. Conventional heart failure therapy, including diuretics, was not utilized given that the suspected underlying cause was thyroid storm.
By day 2 of admission, the patient’s oxygen requirements had decreased, and oxygen was completely weaned by day 3. She was discharged 10 days after hospital admission with a plan for total thyroidectomy upon reaching a euthyroid state. Discharge prescriptions included PTU, propranolol, Lugol’s iodine, and prednisolone.
On endocrinology follow-up 2 weeks after discharge, the patient was clinically stable with normal vital signs, clear lungs to auscultation, and resolution of peripheral edema. Her bedside ECHO demonstrated a normal EF. Repeat laboratory testing revealed normalization of her TSH and free T3 (T4 testing was unavailable). The patient was subsequently referred to endocrinology for further care.
Take-Home Points
- Thyroid storm is a potentially fatal manifestation of untreated thyrotoxicosis and may result in cardiopulmonary dysfunction.
- Cardiopulmonary failure is potentially reversible with prompt antithyroid therapy.
- Physicians should have a low threshold to treat and test for thyrotoxicosis in young patients with new-onset heart failure, as early treatment has the potential to improve patient outcomes.
References
- De Filippis EA, Sabet A, Sun MRM, Garber JR. Pemberton’s Sign: Explained Nearly 70 Years Later. J Clin Endocrinol Metab. 2014;99(6):1949-1954.
- Galindo RJ, Hurtado CR, Pasquel FJ, García Tome R, Peng L, Umpierrez GE. National Trends in Incidence, Mortality, and Clinical Outcomes of Patients Hospitalized for Thyrotoxicosis With and Without Thyroid Storm in the United States, 2004–2013. Thyroid. 2019;29(1):36-43.
- Sarlis NJ, Gourgiotis L. Thyroid Emergencies. Rev Endocr Metab Disord. 2003;4(2):129-136.
- Idrose AM. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg. 2015;2(3):147-157.
- Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691-700.
- Fadel BM, Ellahham S, Lindsay J, Ringel MD, Wartofsky L, Burman KD. Hyperthyroid heart disease. Clin Cardiol. 2000;23(6):402-408.
- Fadel BM, Ellahham S, Lindsay J, Ringel MD, Wartofsky L, Burman KD. Hyperthyroid heart disease. Clin Cardiol. 2000;23(6):402-408.
- Goland S, Shimoni S, Kracoff O. Dilated cardiomyopathy in thyrotoxicosis. Heart. 1999;81(4):444-445.
- Dahl P, Danzi S, Klein I. Thyrotoxic cardiac disease. Curr Heart Fail Rep. 2008;5(3):170-176.
- Al-Ghamdi AS, Aljohani N. Graves’ Thyrotoxicosis-Induced Reversible Cardiomyopathy: A Case Report. Clin Med Insights Case Rep. 2013;6:CCRep.S10534.
- Mier A, Brophy C, Wass JAH, Besser GM, Green M. Reversible Respiratory Muscle Weakness in Hyperthyroidism. Am Rev Respir Dis. 1989;139(2):529-533.
- Kazakov VM. Terminal intramuscular motor innervation and motor end-plates in thyrotoxic myopathy. Neuromuscul Disord. 1992;2(5-6):343-349.
- Mehta PA, Dubrey SW. High output heart failure. QJM. 2009;102(4):235-241.
- Johnson CR, Fischer PR, Thacher TD, Topazian MD, Bourassa MW, Combs GF. Thiamin deficiency in low- and middle-income countries: Disorders, prevalences, previous interventions and current recommendations. Nutr Health. 2019;25(2):127-151.
- Siu CW, Yeung CY, Lau CP, Kung AWC, Tse HF. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93(4):483-487.
- Ali A, Mostafa W, Fernandez C, Ahmad H, Htwe N. Apathetic Thyroid Storm with Cardiorespiratory Failure, Pulmonary Embolism, and Coagulopathy in a Young Male with Graves’ Disease and Myopathy. Case Rep Endocrinol. 2020;2020:1-9.
- Kiriyama H, Amiya E, Hatano M, et al. Rapid Improvement of thyroid storm-related hemodynamic collapse by aggressive anti-thyroid therapy including steroid pulse: A case report. Medicine (Baltimore). 2017;96(22):e7053.
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
- Fotso Simo SC, Lin KS, Bukharovich I. Thyrotoxic Cardiomyopathy: A Rare Case of Thyroid Storm Presenting as De Novo Heart Failure. Cureus. Published online April 9, 2024.
- Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: Improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun Rev. 2012;12(2):107-113.
- Vaidya B, Pearce SHS. Diagnosis and management of thyrotoxicosis. BMJ. 2014;349(aug21 7):g5128-g5128.
- Jeyamalar R, Chan SP. A reversible dilated cardiomyopathy due to thyrotoxicosis. Int J Cardiol. 1995;52(1):83-84.
- Nai Q, Ansari M, Pak S, et al. Cardiorespiratory Failure in Thyroid Storm: Case Report and Literature Review. J Clin Med Res. 2018;10(4):351-357.
- Raguthu CC, Gajjela H, Kela I, et al. Cardiovascular Involvement in Thyrotoxicosis Resulting in Heart Failure: The Risk Factors and Hemodynamic Implications. Cureus. Published online January 13, 2022.
- N J, Francis J. Atrial fibrillation and hyperthyroidism. Indian Pacing Electrophysiol J. 2005;5(4):305-311.
- Carroll R, Matfin G. Review: Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab. 2010;1(3):139-145.
- Barreto-Chaves MLM, Carrillo-Sepúlveda MA, Carneiro-Ramos MS, Gomes DA, Diniz GP. The crosstalk between thyroid hormones and the Renin–Angiotensin System. Vascul Pharmacol. 2010;52(3-4):166-170.