
Post-MI ventricular septal rupture is a rare but life-threatening complication. Prompt diagnosis and surgical intervention are crucial for improving outcomes. This case highlights the speed, sensitivity, and specificity ultrasound offers in developing a treatment plan.
Case
A previously healthy 65-year-old woman presents to the emergency department with dizziness and lightheadedness. The patient’s symptoms began 3 days prior with fatigue and lightheadedness. Prior to this presentation, the patient infrequently followed up with any medical professional, as she did not have health insurance. The patient had a history of daily tobacco use, but no history of chronic conditions.
On arrival the patient was noted to have tachycardia and mild tachypnea. Her exam was notable for delayed capillary refill, narrow pulse pressure, and a holosystolic murmur. Lab work shows a lactic acid of 2.6, leukocytosis of 18.45, and a high sensitivity troponin of 2268. The patient’s lab work was otherwise at her baseline.
Chest X-ray showed borderline cardiomegaly and probable pulmonary hypertension. An EKG was performed which showed an ST elevation in the anteroseptal leads along with Q-waves and sinus tachycardia (Image 1). A STEMI alert was called and a bedside ultrasound was performed (Images 2 and 3).
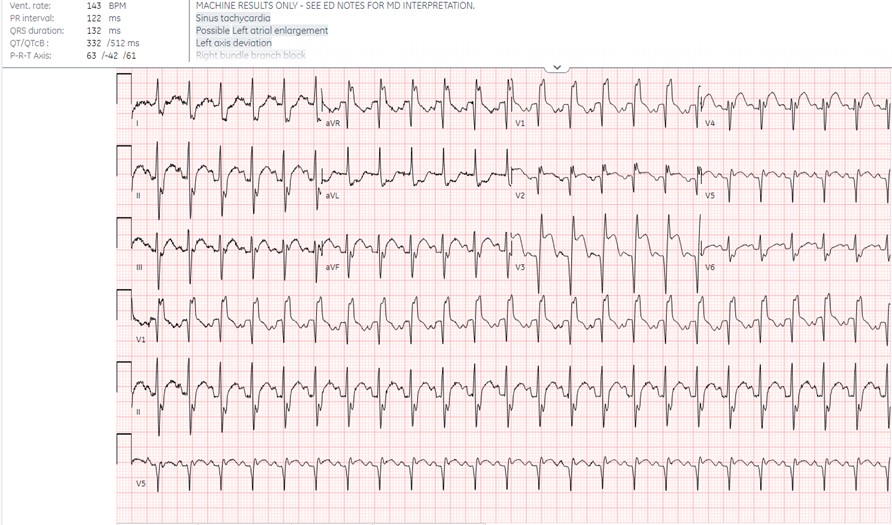
Image 1. EKG shows sinus tachycardia with a right bundle branch block and left anterior fascicular block. There are ST elevations and q-waves in the anteroseptal leads.
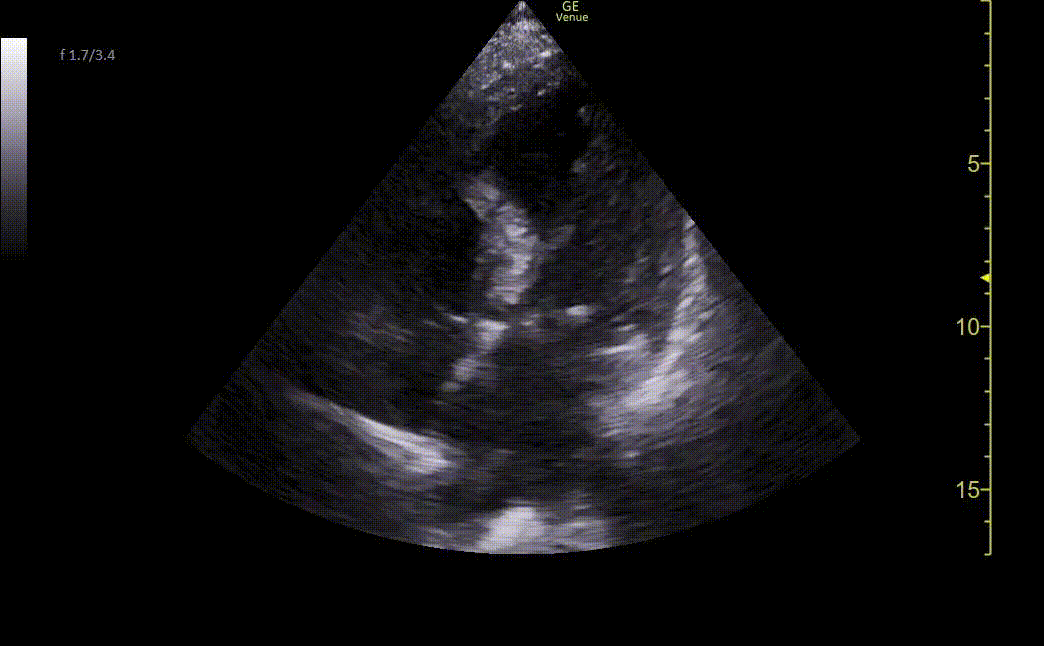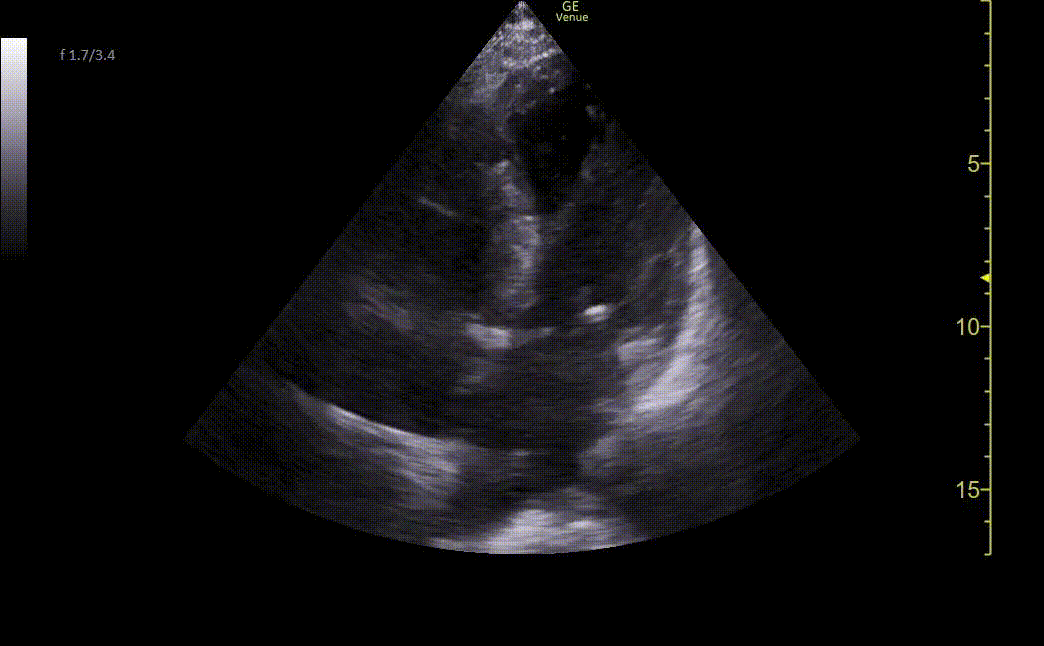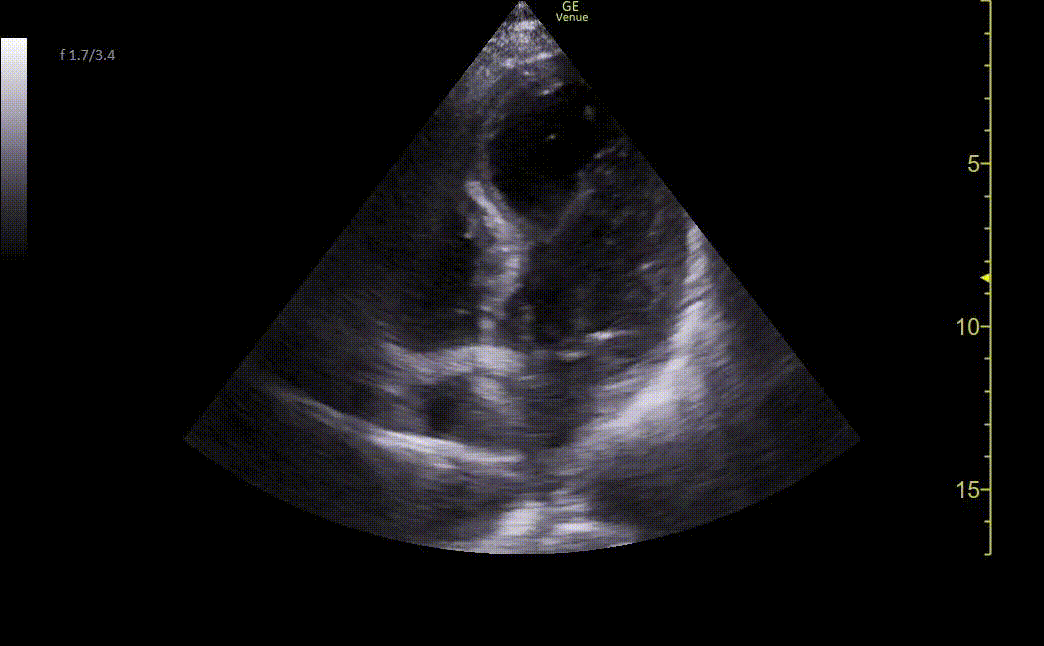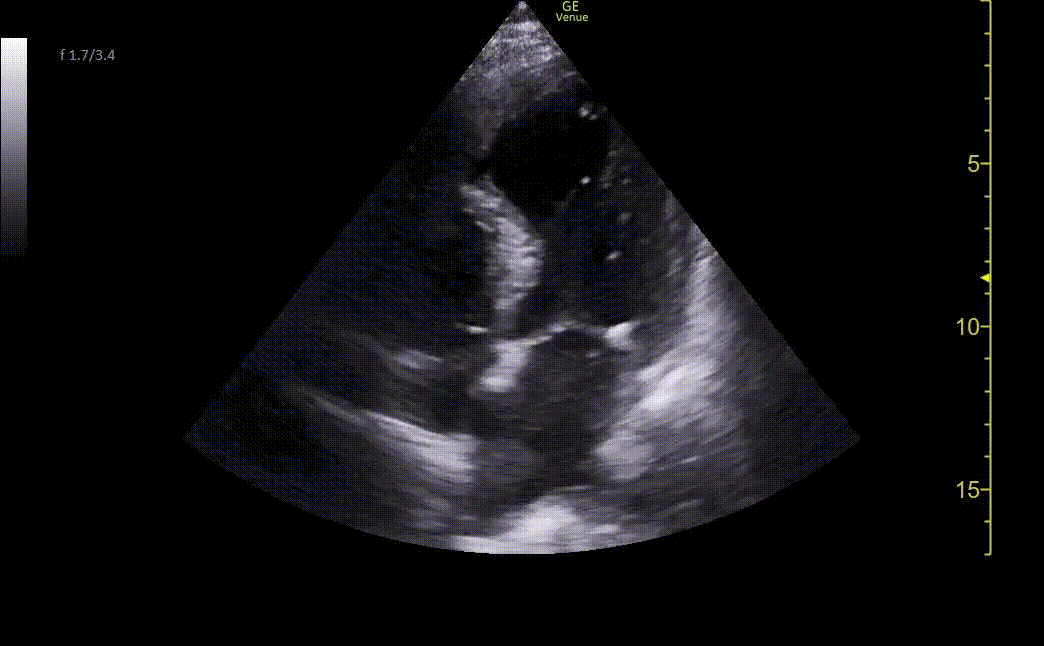
Image 2. Ultrasound shows an apical four-chamber view. There is a moderate decrease in ejection fraction. Regional wall motion abnormalities can be seen with severe hypokinesis of the mid-apical anteroseptal wall and the entire apex. Mid-apical septal post-MI VSD is noted with a defect measuring up to 2.2 cm at the widest opening on the LV side. Left apical thrombus can also be seen.
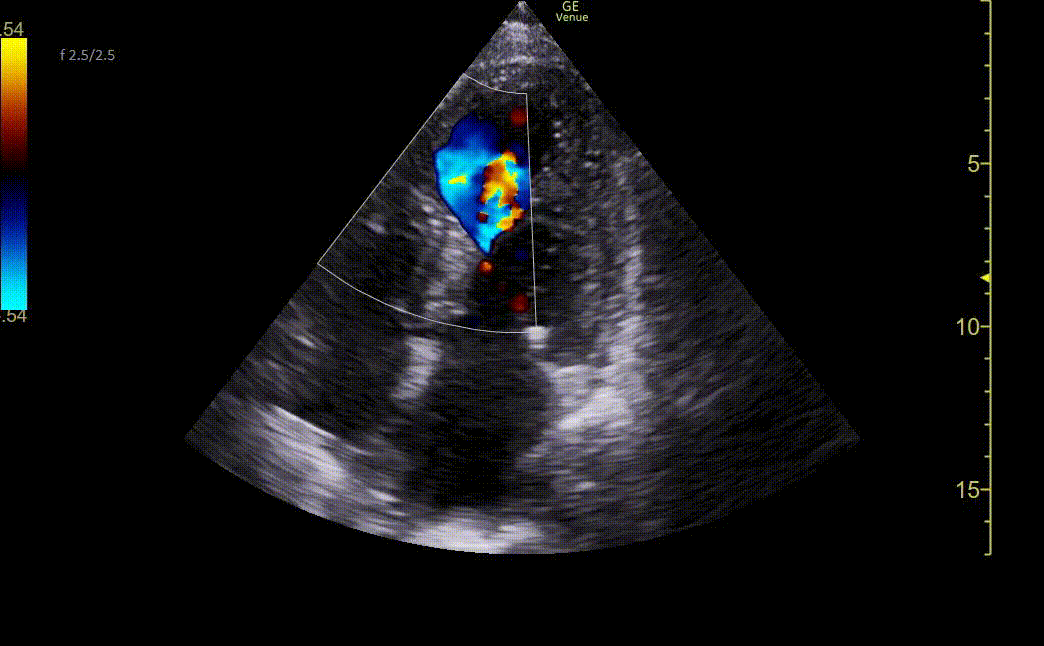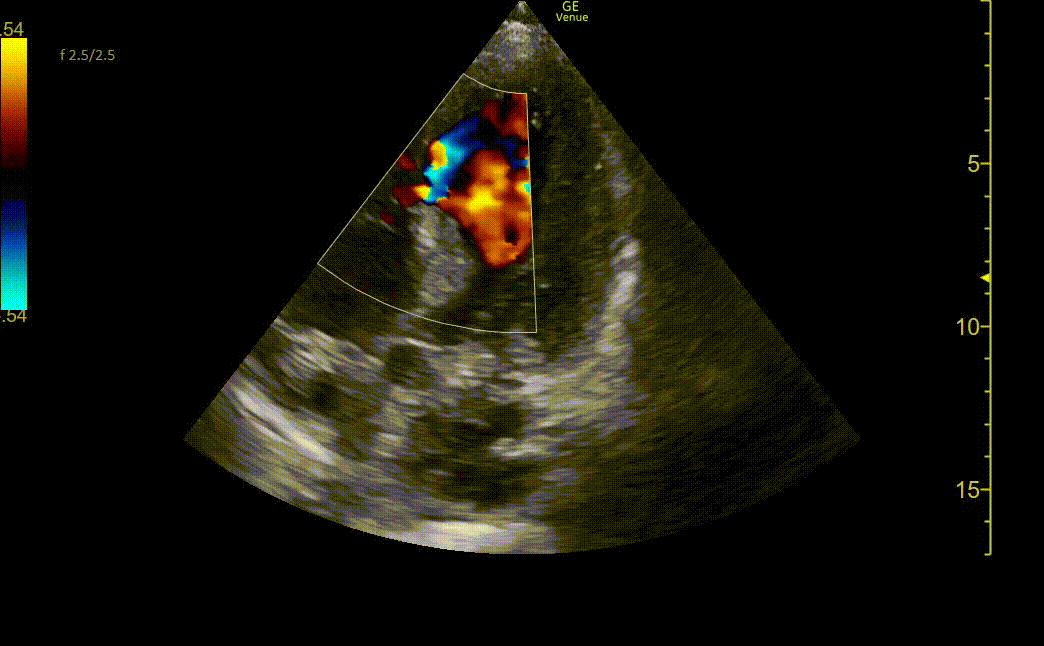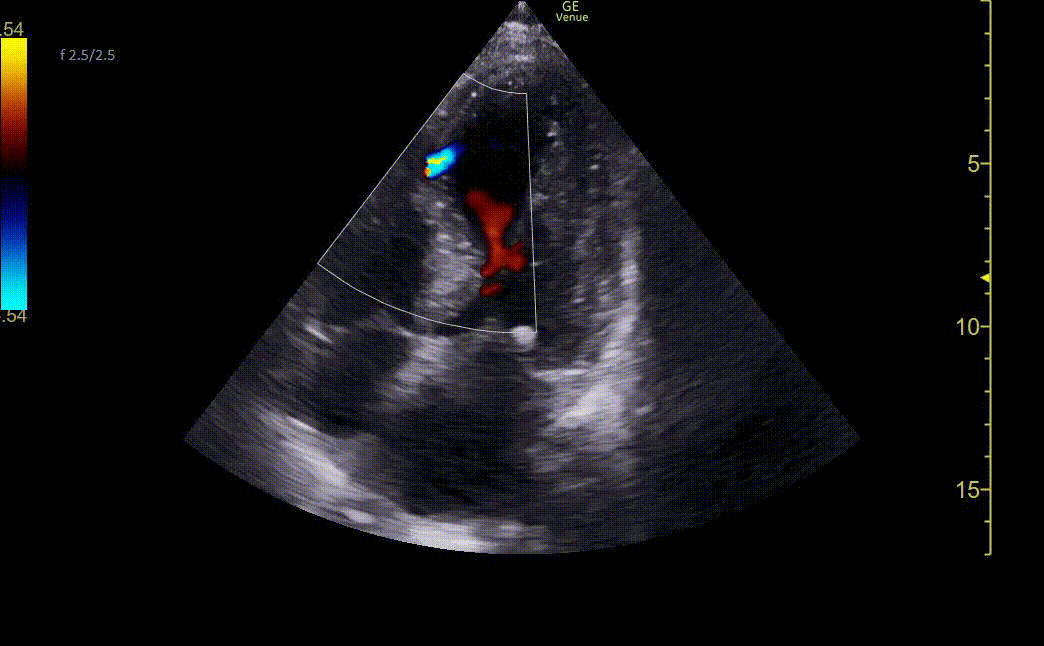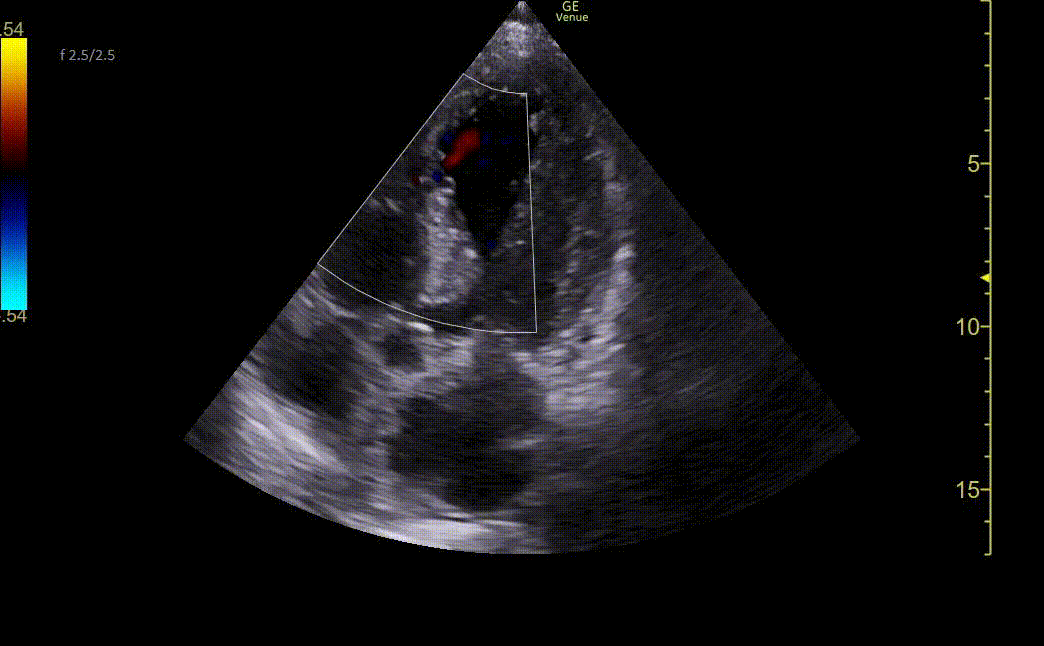
Image 3. Ultrasound showing mid-apical septal post-MI VSD with left-to-right flow on color Doppler. There is also severe hypokinesis of the entire apex and mid-apical anteroseptal wall.
Diagnosis: Post-Myocardial Infarction Interventricular Septum Rupture
Discussion
The interventricular septum receives its blood flow from the left anterior descending artery, the dominant right coronary artery, or less commonly the dominant left-circumflex arteries. Infarction at this location often will become transmural and is at risk for rupture. This rupture will occur in the anteroseptal wall two-thirds of the time and in the inferior or posterior wall one-third of the time. Rupture near the posterior inferior wall can lead to mitral valve insufficiency due to papillary muscle rupture.1 The diminished blood supply leads to a coagulation necrosis of the septal tissue, which will advance toward thinning of the septal wall and eventual rupture. This process typically takes 3-8 days. A rupture will create a communication between the two ventricles, which creates sudden volume overload and can lead to pulmonary edema (Image 4).
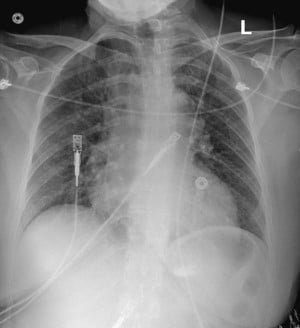
Image 4. Chest X-ray on arrival with prominent central pulmonary vasculature concerning for pulmonary hypertension
Ventricular septal rupture occurs after approximately 0.21% of acute myocardial infarctions and carries a 40% mortality with operative management.2 Ventricular septal rupture can be found with auscultation, but point-of-care ultrasound is the preferred method for confirming this diagnosis. Prompt diagnosis is critical and associated with improved outcomes.3 POCUS has been shown to rapidly assist in diagnosing this pathology. Ultrasound will also allow the provider to evaluate for other more common findings associated with myocardial infarction, including wall motion abnormalities and general cardiac function. Cardiac thrombus can also be seen in these patients, as they will have an increased risk for formation post-rupture.
The POCUS findings typically associated with septal rupture include direct visualization of the defect, right ventricle dilation, and color flow across the interventricular septum. This may require sweeping across the septum.4 Ultrasound was found to be 90% sensitive and 98% specific for this diagnosis.5 POCUS also allows for the evaluation of other causes of shock.
Management
Treatment for a septal rupture will require coordination with multiple disciplines including interventional cardiology and cardiothoracic surgery. The ultimate goal will be surgical repair of the defect. Surgical intervention is required for management, but at what time this intervention occurs is up for debate. Early surgery significantly increased mortality in some studies when compared to delayed surgery. Those patients with significant shunting and hemodynamic compromise are still recommended for early surgical repair.6
Initial management includes using vasodilators to decrease afterload on the heart, with the goal of decreased left-to-right shunting. The patient will likely be hypotensive, and vasopressors can be considered, but this intervention will lead to increased afterload and can be associated with worse outcomes. Intra-aortic balloon pump is considered the preferred temporizing measure as this will decrease afterload, decrease shunting, and facilitate coronary perfusion. More recent studies show VA-ECMO is a reasonable intervention to decrease multiorgan failure and can possibly improve mortality.7
Post-MI ventricular septal rupture is a rare but life-threatening complication. Prompt diagnosis and surgical intervention are crucial for improving outcomes. This case emphasizes the importance of considering such complications in patients, even without traditional cardiovascular risk factors.
Case Conclusion
Interventricular septal wall rupture was seen on POCUS. EKG showed a STEMI with Q-waves present. Based on her timeline, she likely experienced a myocardial infarction 3 days prior to presentation. Interdisciplinary discussions with cardiology and cardiothoracic surgery led to an initial plan for catheterization lab activation for revascularization. Emergent coronary angiography demonstrated a complete occlusion of the LAD and distal right coronary artery. An intra-aortic balloon pump was placed.
Upon return to the Cardiac Care Unit, the patient became unresponsive with tachycardia and hypoxia. There was concern for right-to-left shunting and VA-ECMO was initiated. The following day the patient went to the OR for VSD closure and left ventricular thrombus evacuation. The patient was eventually weaned off pressors and decannulated from ECMO, but unfortunately suffered a stroke, leading to a poor neurologic outcome. The patient was discharged to a long-term acute care hospital.
References
- Mubarik A, Iqbal AM. Ventricular Septal Rupture. [Updated 2022 Sep 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- David TE. Post-infarction ventricular septal rupture. Ann Cardiothorac Surg. 2022 May;11(3):261-267.
- Mahajan K, Shah N, Patel H. Postinfarction Ventricular Septal Rupture. [Updated 2022 Sep 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Mukherjee A, Fong J. Heartbreak: a case of post-infarction cardiogenic shock. Australas J Ultrasound Med. 2019;22(1):66–71.
- Triposkiadis F, Xanthopoulos A, Boudoulas KD, Giamouzis G, Boudoulas H, Skoularigis J. The Interventricular Septum: Structure, Function, Dysfunction, and Diseases. J Clin Med. 2022;11(11):3227.
- Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular septal rupture? Interact Cardiovasc Thorac Surg. 2013;16(2):193-196.
- Rob D, Špunda R, Lindner J, et al. A rationale for early extracorporeal membrane oxygenation in patients with postinfarction ventricular septal rupture complicated by cardiogenic shock. Eur J Heart Fail. 2017;19(S 2):97-103.



