Naloxone is a potent opioid receptor antagonist with an excellent safety profile and ability to reverse opioid-associated respiratory depression. Is it as effective when you're faced with patients in cardiac arrest secondary to an overdose?
On an EMS rotation, you are dispatched to a call for a 26-year-old male, cardiac arrest – CPR in progress. On arrival, you note a man supine on the porch of a rundown house. He is apneic and pulseless, skin is ashen. No rigor is noted. A bystander on scene is attempting to perform CPR by 911 dispatch instructions, but he appears intoxicated. Paramedics take over CPR, placing the patient on their cardiac monitor. His initial rhythm is asystole, but after two rounds of epinephrine converts to pulseless electrical activity. He is intubated, with an EtCO2 of 23. While paramedics continue to work the code, bystanders state that the patient was recently released from jail and was using heroin, and that it was a “bad batch.” They used half an hour prior and found their friend unresponsive upon awakening. The paramedics turn to you for medical direction – should they administer naloxone?
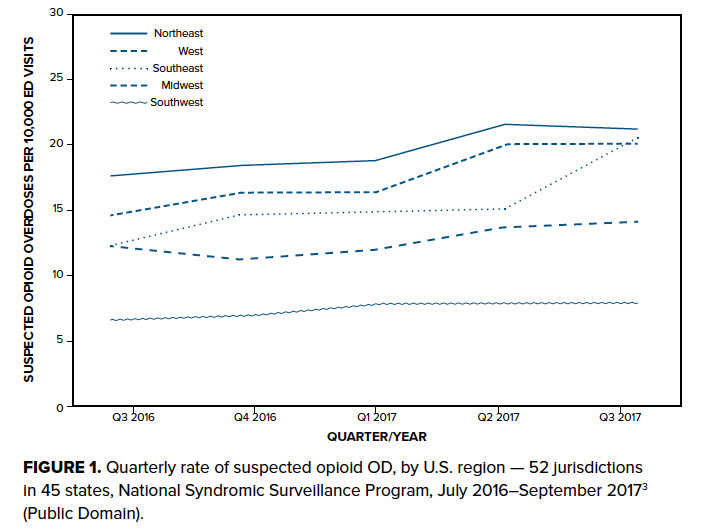
The Centers for Disease Control and Prevention found that more than 70,000 people died from drug overdose (OD) in the United States in 2017 - more than those who died from gun violence or motor vehicle collisions.1 More than 47,000 died from opioid overdose, with approximately 28,000 involving synthetic opioids (other than methadone). This staggering rise in mortality corresponds to increased access to powerful synthetic opioids such as fentanyl. Fentanyl began trickling into the illicit drug supply a decade ago, but many municipalities have found fentanyl-associated deaths now exceeding those due to oxycodone and heroin.2 National mortality due to fentanyl nearly doubled from 2016 alone. 1 Nationally, opioid overdose ED visits increased by nearly 30% from the third quarter of 2016 to the third quarter of 2017, even more so in the U.S. Southeast3 (Figure 1). One large public hospital in Miami saw a nearly fivefold increase in naloxone use between 2015 and 2016,4 while EMS use of naloxone in the same county increased more than tenfold.5
The main approach to combating this overwhelming rise in mortality has been through needle exchange programs, health departments, and even hospital emergency departments and EMS distributing naloxone to people at greatest risk of witnessing or experiencing an opioid OD.6,7 Previously a medication only available in the medical setting, increasing naloxone availability has complicated Good Samaritan resuscitation for the layperson rescuer when faced with an unresponsive patient.
The 2015 American Heart Association (AHA) Emergency Cardiac Care (ECC) guidelines address this complexity directly, recommending that despite a lack of evidence that “administration of naloxone will help a patient in cardiac arrest… empiric administration of IM or IN naloxone to all unresponsive opioid-associated resuscitative emergency patients” may be a reasonable adjunct to standard first aid and non-healthcare provider basic life support protocols, but that “CPR should take precedence over naloxone administration as patients without a palpable pulse may be in cardiac arrest or may have an undetected weak or slow pulse.”8
While instructive for the layperson rescuer, this guideline references a historical conundrum surrounding naloxone use in patients suffering cardiac arrest secondary to opioid OD. For professional rescuers in either the prehospital or hospital settings, naloxone remains a constituent of the AHA Advanced Cardiac Life Support (ACLS) Algorithm and is found in the list of interventions to correct reversible causes of cardiac arrest as an adjunct to correct the hypercarbic respiratory failure caused by opioid OD. Although naloxone’s efficacy in treating respiratory depression in patients with a pulse is well established, its utility in cardiac arrest remains controversial.
History of Naloxone in Cardiac Arrest
Prior to the advent of EMS equipped with ventilatory equipment and naloxone, respiratory failure in the out of hospital setting frequently resulted in death.9 µ-opioid receptor agonists such as morphine, heroin, and fentanyl induce significant respiratory depression, responsible for the toxicity of opioid OD. Naloxone was developed in 1961 and has played a varying role in the resuscitation of opioid OD victims once the drug became available a decade later.10 Heroin and other opiates were responsible for significant morbidity and mortality in the first half of the 20th century.11 However, the earliest iterations of ACLS referenced naloxone only for use in the resuscitation of neonates with respiratory depression “induced by narcotics given to the mother before delivery.”9,12 It was not until the 2000 ECC guidelines that opioid OD was recognized in ACLS as a “pre-arrest poison” with the recommendation to “try to reverse respiratory insufficiency with naloxone before inserting an endotracheal tube.”13 In the years since, an epidemic of opioid OD has overtaken the United States, making naloxone one of the most important drugs in the prehospital pharmacopeia.
Many studies and practice guidelines have recommended the use of naloxone in cardiac arrest patients. However, in the 2015 ACLS guidelines, the AHA endorses specific indications for empiric naloxone administration in the peri-arrest setting:
- “It may be reasonable to administer IM or IN naloxone based on the possibility that the patient is not in cardiac arrest. (Class IIb, LOE C-EO)
- Standard resuscitative measures should take priority over naloxone administration, with a focus on high-quality CPR (compressions plus ventilation). (Class I, LOE C-EO)
- We can make no recommendation regarding the administration of naloxone in confirmed opioid-associated cardiac arrest. Patients with opioid-associated cardiac arrest are managed in accordance with standard ACLS practices.”14
In the absence of a clear recommendation on naloxone in confirmed cardiac arrest patients, physicians are left to the literature base to support decision-making when faced with patients in cardiac arrest secondary to presumed or known opioid OD.
Evidence Supporting Naloxone Use in Cardiac Arrest
Naloxone is a potent opioid receptor antagonist with an excellent safety profile and ability to reverse opioid-associated respiratory depression in patients. It can be administered via intravenous, intraosseous, intramuscular, subcutaneous or intranasal routes, as well as nebulized for inhalation. There are few adverse events associated with naloxone. The most prominent adverse event is the precipitation of acute withdrawal, with signs and symptoms including agitation, hypertension, tachydysrhythmias, and vomiting.15,16 Precipitated withdrawal is particularly dangerous in opioid overdoses in the setting of polypharmacy overdose.
Reversal of opioid overdose in the presence of stimulants can trigger unopposed catecholaminergic activity with subsequent demand ischemia. Reversal of overdose in the presence of CNS depressants is particularly dangerous as patients who remain obtunded but with an iatrogenically induced predisposition for emesis are at increased risk for aspiration. Although several reports exist in the literature describing flash non-cardiogenic pulmonary edema occurring following naloxone administration, this phenomenon is rare at a rate of 0.2-3.6% in patients transported to ED following opioid overdose.17 Documented since the 1970s, the etiology of naloxone associated non-cardiogenic pulmonary edema is still poorly understood and potentially caused by rapidly stimulated ventilatory drive in the presence of a closed glottis or a sequelae of the histaminergic activity of opioid use itself.18
Theoretically, administration of naloxone prior to ROSC could result in a patient who can protect their own airway upon obtaining a pulse, thereby abrogating the need for aggressive airway management, including intubation.
Several case reports and animal studies describe antiarrhythmic and positive inotropic effects of naloxone in cardiac arrest. 19,20 Likewise, a retrospective chart review of emergency medical dispatches for cardiac arrest in which patients received naloxone found that 42% of patients who received naloxone in the prehospital setting had an improvement in electrocardiogram (EKG). Furthermore, “of the participants who responded to naloxone, 47% demonstrated EKG rhythm changes immediately following the administration of naloxone.” These rhythm changes were inconsistent and included sinus tachycardia, accelerated junctional rhythm, PEA, ventricular tachycardia and ventricular fibrillation.21
Two other studies on demographics of out-of-hospital cardiac arrests (OHCA) found that patients in cardiac arrest secondary to a presumed opioid OD had improved odds of survival to ED and survival to hospital discharge. The first found an odds ratio of 1.21 (P=0.66) for survival to ED, while the other found a higher rate of survival to hospital discharge (19% vs. 12%, p=0.014) versus non-overdoses.22,23 These findings support the recommendation to administer naloxone in opioid associated OHCA, because given “low rates of [ROSC] and survival during cardiac arrest, any potential intervention leading to rhythm improvement is a reasonable treatment modality.”21 Baseline prognostic factors in cardiac arrest secondary to opioid overdose are poor and outcomes are often dismal. However, this is not an acceptable reason to limit use of a pharmacologic agent with potential mortality benefit: A review of patients who survived OHCA secondary to OD with or without naloxone found that outcomes were “no worse than after non-OD OHCA, and among survivors a majority had a good neurological outcome.”22
Evidence Opposing Naloxone Use in Cardiac Arrest
While the results of the previous studies are compelling, it is important to consider the strength of the evidence. The pharmacological data underlying a purported efficacy of naloxone in achieving ROSC is ambiguous, and contradictory: the animal model demonstrating higher rates of ROSC in the presence of naloxone also noted ROSC duration to be 1.5 times shorter versus epinephrine alone or saline.19 While groundbreaking as the first human cohort observed, Saybolt et al.’s results are low-powered and a prospective trial with fewer confounders would be necessary to demonstrate effect. Although in many cases observed rhythm changes occurred immediately following naloxone administration, the number of pharmacologic agents involved in ACLS, each with distinct pharmacokinetics, makes causality of the naloxone intervention difficult to establish. Likewise, within the epidemiologic literature, there are many confounding factors that may have played a greater role in opioid-associated OHCA patient outcomes; specifically, OD OHCA patients were on average significantly younger, had fewer medical comorbidities, were more likely to present with non-shockable rhythms and had worse baseline neurological function as measured by Glasgow Coma Scale. 22 Further, one of the demographic studies notes that among OD OHCA patients, 40 (47%) received naloxone and naloxone use was not associated with survival (P = 0.54).
The AHA asserts that in most settings, it is difficult to establish that the patient’s “clinical condition is due to opioid induced CNS and respiratory depression toxicity alone, and [providers] might therefore misidentify opioid-associated cardiac arrest as unconsciousness or vice versa… particularly where determination of the presence or absence of a pulse is unreliable.” 8 This difficulty may further confound providers when considering administration of naloxone, as what may have appeared to be an effective treatment for cardiac arrest merely reversed severe CNS depression. While naloxone is an uncertain treatment in patients without a pulse, it is clear is that naloxone reverses respiratory depression in patients with a pulse. A patient in cardiac arrest due to apparent OD overdose should be treated for a respiratory cause of cardiac arrest. The treatment for respiratory failure centers around airway management, which necessitates controlling the patient's oxygenation and ventilation. This may be achieved via endotracheal intubation or placement of a supraglottic airway to ensure adequate ventilation and oxygenation. Given the many harms associated with intubation, it may be prudent to reserve placement of an advanced airway until later in a resuscitative effort. Empiric naloxone use does not improve oxygenation or ventilatory drive in a pulseless patient, and as such does not improve not add anything to the treatments the patient is already receiving. By placing a focus on rapid administration of naloxone in cardiac arrest patients with presumed opioid OD, providers may be predisposed providers to diagnostic inertia and distracted from evidence-based methods to improve care in cardiac arrest: namely, early recognition, high quality CPR, and early defibrillation.
Conclusions
With the rapid rise in opioid-associated deaths and widespread dissemination of naloxone, emergency care professionals are faced with the decision whether or not to administer naloxone to patients in cardiac arrest after a presumed OD. With scant evidence to support or refute the empiric use in opioid OD-associated cardiac arrest, health care professionals should tailor their approaches to cardiac arrest to the etiology of arrest, rather than empiric intervention. Even so, the evidence supporting naloxone may point towards a possible role for the drug. Further investigation, including a prospective clinical trial, would be necessary to investigate this role. In the meantime, it is critical to focus attention to the treatments we know work best.
Case Conclusion
You recommend against administering naloxone because the patient is already intubated. Following the third round of epinephrine, the patient develops a perfusing rhythm with palpable peripheral pulses, and an initial GCS of 3. He is loaded into the ambulance and paramedics transport him to the local hospital ED. After 16 days in the intensive care unit he demonstrates significantly improved mental status. He is extubated and transferred to a rehabilitation floor for physical and occupational therapy.
References
1. Ahmad R, Spencer, Warner, Sutton. Provisional drug overdose death counts. CDC’s National Center for Health Statistics. 2018.
2. Nelson S, Wolf B, Purdy B, et al. Drugs Identified in Deceased Persons by Florida Medical Examiners 2017 Annual Report. Florida: Florida Department of Law Enforcement. 2018.
3. Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital Signs: Trends in Emergency Department Visits for Suspected Opioid Overdoses - United States, July 2016-September 2017. MMWR. 2018;67(9):279-285.
4. Bode AD, Singh M, Andrews J, Kapur GB, Baez AA. Fentanyl laced heroin and its contribution to a spike in heroin overdose in Miami-Dade County. Am J Emerg Med. 2017;35(9):1364-1365.
5. Haden P. “We Can’t Do Without It”: First Responders Pay Soaring Price For Overdose Antidote Naloxone. WLRN2018.
6. Guza M. Pittsburgh EMS leaving overdose-reversal drug with survivors who decline treatment. Pittsburgh Tribune-Review. 2018.
7. del Ninno T, Wilkerson RG, Khoujah D. Take-Home Naloxone: Preventing Death in an Era of Abuse. EM Resident. 2017;44(3):12-13.
8. Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special Circumstances of Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S501-518.
9. Standards for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiac Care (ECC). JAMA. 1974;227(7):833-868.
10. Yardley W. Jack Fishman Dies at 83; Saved Many From Overdose. The New York Times. Dec 14, 2013.
11. Heilman RD, Hahn EF, Fishman J. Narcotic antagonists. 4. Carbon-6 derivatives of N-substituted noroxymorphones as narcotic antagonists. J Med Chem. 1975;18(3):259-262.
12. National Conference on Cardiopulmonary R, Emergency Cardiac C. Standards and guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiac care (ECC). Part VIII: Medicolegal considerations and recommendations. JAMA. 1986;255(21):2979-2984.
13. 2000 Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8: advanced challenges in resuscitation: section 2: toxicology in ECC. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(8 Suppl):I223-228.
14. American Heart Association. Web-based Integrated Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care - Part 10: Special Circumstances of Resuscitation. [press release]. 2015.
15. Gillespie M, Dela Cruz M, Slutsky J. Atrial Fibrillation after Naloxone Administration: A Rare Complication of Opioid Reversal. EM Resident. 2017;44(5):18.
16. Lameijer H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular Tachycardia After Naloxone Administration: a Drug Related Complication? Case Report and Literature Review. Drug Saf Case Rep. 2014;1(1):2.
17. Jiwa N, Sheth H, Silverman R. Naloxone-Induced Non-Cardiogenic Pulmonary Edema: A Case Report. Drug Saf Case Rep. 2018;5(1):20.
18. Flacke JW, Flacke WE, Williams GD. Acute pulmonary edema following naloxone reversal of high-dose morphine anesthesia. Anesthesiology. 1977;47(4):376-378.
19. Wang Y, Gao L, Meng L. Small-dose naloxone combined with epinephrine improves the resuscitation of cardiopulmonary arrest. Am J Emerg Med. 2008;26(8):898-901.
20. Martins HS, Silva RV, Bugano D, et al. Should naloxone be prescribed in the ED management of patients with cardiac arrest? A case report and review of literature. Am J Emerg Med. 2008;26(1):113 e115-118.
21. Saybolt MD, Alter SM, Dos Santos F, et al. Naloxone in cardiac arrest with suspected opioid overdoses. Resuscitation. 2010;81(1):42-46.
22. Elmer J, Lynch MJ, Kristan J, et al. Recreational drug overdose-related cardiac arrests: break on through to the other side. Resuscitation. 2015;89:177-181.
23. Koller AC, Salcido DD, Callaway CW, Menegazzi JJ. Resuscitation characteristics and outcomes in suspected drug overdose-related out-of-hospital cardiac arrest. Resuscitation. 2014;85(10):1375-1379.



