Methemoglobinemia is a rare but serious — yet treatable — hematological condition that can lead to death if not identified in a timely manner.
In methemoglobinemia, the iron in hemoglobin becomes oxidized. In a normal erythrocyte, the ferrous (Fe2+) state allows oxygen to easily offload to oxygenate tissues. However, in methemoglobinemia, the iron on the hemoglobin is oxidized and becomes the ferric (Fe3+) state.
When this happens, the oxygen does not get picked up by the hemoglobin and, therefore, does not oxygenate the tissue. Furthermore, if only a portion of the red blood cell contains the ferric state and the other contains the ferrous state, the ferrous state is still unable to pick up the oxygen as it causes a left shift in the hemoglobin curve (Figure 1). This causes decreased oxygen delivery to the tissues.1
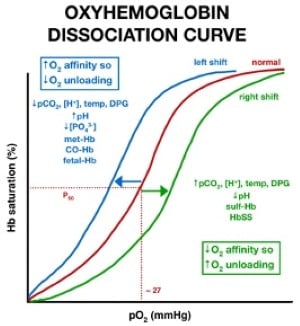
Figure 1: Example of left shift resulting in hemoglobin not being able to pick up oxygen in methemoglobinemia.
Methemoglobinemia can be acquired or hereditary.2 Topical benzocaine is a known cause of acquired methemoglobinemia and is applied for transesophageal echocardiograms, among other procedures and uses. Cyanosis, a low pulse oximetry reading, and a high partial pressure of arterial oxygen on arterial blood gas (ABG) should aid in diagnosis.
Methemoglobinemia is a serious condition and can lead to death. Although benzocaine is known to cause methemoglobinemia, it is still rare.3
In a large case series study, methemoglobinemia occurred in 1 out of 1,499 transesophageal echocardiograms (TEE). In another study — a random sample of 190 patients with the same sedation, sex, body mass index, and left ventricular systolic function — patients who developed methemoglobinemia were found to be anemic, hospitalized, or with acute systemic infection.3
An ABG is crucial in diagnosing methemoglobinemia and should be the first test. Most ABGs can detect methemoglobinemia by its absorbance spectrum of 631 nanometers (expressed as a percentage).
Partial pressure of arterial oxygen (pO2) is an indicator of how much oxygen is being delivered to the tissue and how much dissolved oxygen is in the blood. Methemoglobinemia causes a falsely elevated pO2.4
Pulse oximetry cannot determine how hypoxic a patient truly is in methemoglobinemia. The wavelength of the pulse oximetry can absorb the light of methemoglobinemia and is unable to determine if red blood cells (RBC) are saturated or not.5 An oxygen saturation of 85% is caused by a high percentage of methemoglobinemia.6
A retrospective series, performed in 2 teaching hospitals involving 138 patients with acquired methemoglobinemia, revealed the majority were caused by dapsone. However, 5 of the participants were, in fact, associated with 20% benzocaine spray with a mean methemoglobinemia level of 43.8%. Furthermore, it was shown that 94% of all the subjects in this study were anemic.7
Case Report
The patient in our case was a 76-year-old Caucasian woman with multiple comorbidities, including a history of type 1 diabetes and a lumbar laminectomy 2 months prior. She presented to the ED with recurrent surgical site pain and a fever of 101 degrees Fahrenheit. Her previous laminectomy was complicated by methicillin-resistant staphylococcus aureus (MRSA), necessitating incision and drainage and treatment with intravenous daptomycin for 6 weeks. Blood cultures were obtained, and the patient was discharged due to negative workup in the ED. Two days later, the blood cultures were found to be positive for MRSA, and she was called to return to the hospital.
The patient was admitted that day for bacteremia and diabetic ketoacidosis (DKA). DKA was effectively treated with intravenous (IV) insulin and IV fluids, and it resolved 2 days later. Because of the positive MRSA blood cultures, the patient was started on daptomycin twice a day. Infectious disease and orthopedic specialties were consulted to assist in management. An MRI was performed upon the orthopedist’s recommendation. The MRI revealed a deep lumbar fluid collection.
The patient was taken to the operating room 2 days after admission for excisional debridement of lumbar wound. Infectious disease recommended 6 additional weeks of IV daptomycin, followed by an indefinite course for suppression if cultures were sensitive to daptomycin.
A transthoracic echocardiogram was ordered 1 day before the orthopedic surgery and exhibited findings concerning for a small vegetation that was mobile on the posterior leaflet of the mitral valve. As a result, cardiology was consulted. Cardiology recommended a TEE. The patient was taken to the catheterization lab 4 days later for a TEE. There, she received benzocaine spray before the procedure.
After the TEE, she became very cyanotic and was having difficulty breathing, with an O2 saturation of 85-88%. She was placed on bilevel positive airway pressure (BIPAP) ventilation with FiO2 of 100%. At that time, an ABG was drawn, and the blood appeared dark. The ABG showed pH of 7.48, pCO2 of 44.9 mmHg, pO2 of 574 mmHg, and HCO3 of 33.2 nmol/L.
A computed tomography pulmonary angiogram was performed to rule out pulmonary embolism; it was negative. Despite BIPAP of FiO2 of 100%, the patient remained diffusely cyanotic. She was transferred to the ICU, and another ABG — testing specifically for methemoglobin levels — was completed. Her methemoglobin was found to be 42.3% (Figure 2). She received 1 dose, 100 mg (1.5 mg/kg), of methylene blue.
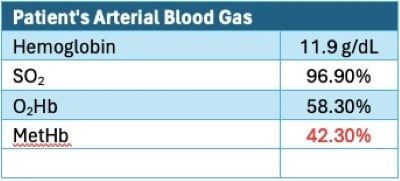
Figure 2: Example of patient’s arterial blood gas.
Another ABG was done 1 hour later. Methemoglobin level was 0.5%, showing improvement. She was taken off BIPAP after the methylene blue infusion, as her hypoxia resolved. She was tested for glucose 6 phosphate dehydrogenase (G6PD) deficiency, which resulted as 262, the normal value being 127-427 U/10E12 red blood cells. TEE showed no endocarditis. The MRSA was found to be sensitive to daptomycin, and this regimen was continued.
The patient remained clinically stable and was moved out of the ICU the following day. Three days later, a peripherally inserted central catheter (PICC) line was placed, and she was discharged home where she would continue to receive daily antibiotic infusions.
Discussion
Determining the cause of methemoglobinemia is important because the cause determines treatment. In fact, first-line therapy is contraindicated in some types of inherited forms.8
In acquired methemoglobinemia, it is important to diagnose promptly and begin treatment immediately to resolve hypoxia. Medications, whether used appropriately or in settings of overdoses, are the main contributors to acquired methemoglobinemia.9
In our case, topical benzocaine was the culprit. However, how benzocaine causes methemoglobinemia isn’t well-known.2
The studies described earlier were similar to our presented case. Oxygen saturation of 85-88%, abnormally high pO2 of 574 mmHg, BIPAP with FiO2 of 100% that did not resolve hypoxia, and dark-appearing blood were all characteristic signs of methemoglobinemia, as seen in the aforementioned studies. Our patient’s methemoglobinemia was 42.3%, similar to the mean values in previous studies, specifically acquired methemoglobinemia after TEE.7 Furthermore, she had bacteremia, which further increased her likelihood of acquiring methemoglobinemia.3 Also, 1 dose of methylene blue caused her to revert to her previous state, further confirming an acquired etiology.
If methemoglobin level is 20-45%, dizziness, dyspnea, tachycardia, coughing, weakness, and headache can occur. If the level is greater than 45%, decreased levels of consciousness and fatigue can occur. When above 55%, cardiac arrhythmias or failure, acidosis, seizure, and coma can occur. If 70% is reached, death can occur.
First-line treatment (if levels are above 30%) is 1-2 mg/kg of IV methylene blue.10 Methylene blue reduces the oxidized state of hemoglobin back to the ferrous state (Fe2+), allowing the hemoglobin to readily pick up oxygen again, resulting in offloading to tissues.
Vitamin C works in the same manner and can be used to treat if methylene blue is contraindicated. Some contraindications for methylene blue are G6PD deficiency because it can cause hemolytic anemia, and pregnancy (category X) because it can lead to intestinal atresia and fetal death, especially if given in the second trimester.11 Furthermore, G6PD deficiency should be considered if the patient does not improve after the first dose of methylene blue. An exchange transfusion can be considered if methylene blue does not resolve methemoglobinemia.10
Because acquired methemoglobinemia is a serious condition caused by an array of medications,9 emergency physicians as well as other care providers should know how methemoglobinemia presents, and all should be familiar with the treatment of choice.
Conclusion
Methemoglobinemia — a serious but treatable condition that presents with generalized cyanosis, an oxygen saturation percentage of mid- to high 80s, high pO2, dark-colored blood, and hypoxia resistant to 100% oxygen — can result from topical benzocaine. It is important to consider methemoglobinemia and perform an ABG if a patient presents similarly. If such signs are present along with a methemoglobin level of 30% or greater, and methemoglobinemia is determined to be acquired, then methylene blue is the treatment of choice.
Take-Home Points
- Consider methemoglobinemia if oxygen saturation level is mid- to high 80s, cyanosis is not improving with 100% FiO2, and dark-colored blood is present.
- Treat with methylene blue when methemoglobin is 30% or above and methemoglobinemia is determined to be acquired.
- Methylene blue is contraindicated in pregnancy and G6PD deficiency. Vitamin C and exchange transfusions are alternative treatments.
References
- Falkenhahn M, Kannan S, O'Kane M. Unexplained acute severe methaemoglobinaemia in a young adult. Br J Anaesth. 2001; 86(2):278-280. doi: 10.1093/bja/86.2.278.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: A review and recommendations for management. J Cardiothoracic Vascular Anesthesia. 2014; doi: 10.1053/j.jvca.2013.02.005.
- Kane GC, Hoehn SM, Behrenbeck TR, et al. Benzocaine-induced methemoglobinemia based on the Mayo Clinic experience from 28,478 transesophageal echocardiograms: Incidence, outcomes, and predisposing factors. Arch Intern Med. 2007 Oct 8;167(18):1977-82. doi: 10.1001/archinte.167.18.1977.
- Cefalu JN, Joshi TV, Spalitta MJ, et al. Methemoglobinemia in the Operating Room and Intensive Care Unit: Early recognition, pathophysiology, and management. advanced therapy. 2020;37(5):1714-1723. doi: 10.1007/s12325-020-01282-5.
- Mack E. Focus on diagnosis: co-oximetry. Pediatric Review. 2007;28(2):73-74. doi: 10.1542/pir.28-2-73.
- Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology. 1989;70(1):112-7. doi: 10.1097/00000542-198901000-00021.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: A retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore). 2004;83(5):265-273. doi: 10.1097/01.md.0000141096.00377.3f.
- Rianprakaisang T, Blumenberg A, Hendrickson RG. Methemoglobinemia associated with massive acetaminophen ingestion: A case series. Clin Toxicol (Phila). 2020; 58(6):495-497. doi: 10.1080/15563650.2019.1657883.
- Hartshorn EA, Rodriguez LF, Smolik LM, et al. Benzocaine-Induced Methemoglobinemia: Report of a Severe Reaction and Review of the Literature. Annals of Pharmacotherapy. 1994;28(5):643-649. doi:10.1177/106002809402800515
- David RS, Sawal NS, Hamzah MN, et al. The Blood Blues: A review on methemoglobinemia. Journal of Pharmacology and Pharmacotherapeutics.2018;9(1):1-5. doi: 4103/jpp.JPP_79_17
- Bistas E, Sanghavi DK. Methylene Blue. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 26, 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557593/
- Kumar, R. Oxyhemoglobin Dissociation Curve.MD Published April 16, 2017. Accessed May 2024. https://rk.md/2017/oxyhemoglobin-dissociation-curve/