While emergency physicians have become more accustomed to managing COVID-19 in adults, the knowledge regarding how the virus affects children still lags behind.
A major concern for the physician is discharging a child who presents with mild upper respiratory or gastrointestinal symptoms only to have the patient return to the ED several days to weeks later, presenting with Multisystem Inflammatory Syndrome in Children (MIS-C). Fortunately, approaching this rapidly progressive and potentially fatal disease process under the larger umbrella of a Kawasaki-Like Hyperinflammatory Syndrome1 can help guide management in a condition where early diagnosis and intervention are crucial to survival.
Case
A 4-year-old previously healthy Hispanic female presented to the ED with a diffuse rash and facial swelling, concerning for an apparent allergic reaction. She was rushed into the treatment area for evaluation of possible anaphylaxis and respiratory assessment. She was tachycardic with a heart rate of 130, tachypneic, and borderline hypotensive for her age. Initial exam was negative for wheezing or stridor, but she had edema of the face and neck with a red maculopapular rash covering her face. Epinephrine IM, diphenhydramine PO, and methylprednisolone IV were given to treat anaphylaxis.
Additional information from the mother revealed no prior history of anaphylaxis, no new food/medication ingestions or topical exposures, no known allergies, and no significant past medical history. Of note, she had been seen in the ED 3 days earlier, diagnosed with strep pharyngitis, and discharged after treatment with IM penicillin G. The patient was tearful, ill-appearing, and withdrawn. On a quick review of systems, mom reports that over the past 5 days her daughter initially had a high fever (102o F) and sore throat, followed by anterior neck swelling on day 2, with vomiting, diarrhea, and the rash presenting on day 4. On secondary examination, the patient was noted to be febrile in the ED, and physical exam was significant for diffuse blanching maculopapular rash, tender cervical lymphadenopathy, dry lips, conjunctivitis, and bilateral hand and foot edema and erythema. While her presentation was most consistent with Kawasaki disease, given the current pandemic, MIS-C was also high on the list of differential diagnoses. Other diagnoses considered included other viral exanthems (EBV, CMV, measles, adenovirus), scarlet fever, toxic shock syndrome, staphylococcal scalded skin syndrome, Stevens-Johnson syndrome, allergic reaction, and sepsis.
ED Course
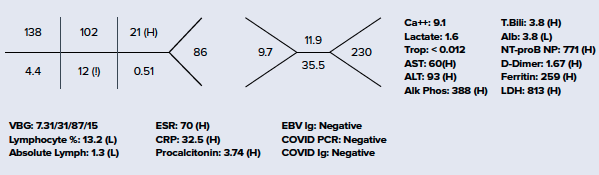
Considering MIS-C as a likely alternative diagnosis, a slower approach to fluid resuscitation was utilized instead of the traditional 20 cc/kg bolus used in sepsis/septic shock, due to risk of development of acute heart failure. Vital signs and repeat physical exam were essentially unchanged following the first 10 cc/kg NS bolus, so an additional 10 cc/kg bolus was administered. Ceftriaxone and vancomycin were also initiated in the ED, with blood and urine cultures pending at the time of admission. EKG showed sinus tachycardia. Chest X-ray was negative for pneumonia or any other acute cardiopulmonary findings. Additional laboratory workup is seen below. She was admitted to the Pediatric ICU for further management of Kawasaki disease vs. MIS-C.
Discussion
Kawasaki Disease (KD) is the second most common vasculitis in children after Henoch-Schönlein Purpura affecting small-to-medium size arteries. It has replaced rheumatic heart disease as the most common cause of acquired heart disease in children in developed countries. The disease was identified by Dr. Tomisaku Kawasaki in the 1960s, who described it as acute febrile mucocutaneous lymph node syndrome. It usually affects children < 5 years old and appears to peak in the months of January and June/July.2 Development of the disease also appears to be influenced by regional and genetic factors, notably in individuals of Asian descent. KD is generally self-limiting and rarely fatal, with an in-hospital mortality rate of only 0.17% in the U.S. Almost all deaths attributed to the disease result from cardiac complications. A significant factor in overall prognosis is the time to diagnosis and initiation of appropriate treatment. Coronary artery aneurysms occur in up to 25% of untreated children and peak mortality is seen in days 15-45 following the onset of fever.3
Despite more than 50 years of studying KD and linking it to a post-infectious process, no definitive cause has been determined.4 Attempts at identifying a viral cause appeared promising when Dr. Kawasaki observed a relative spike in children presenting with symptoms consistent with this syndrome following a coronavirus rhinitis outbreak in Japan. However, later studies provided inconsistent results and failed to establish a link between acutely infected coronavirus patients and KD.5
MIS-C is defined by the CDC as:
- Patients aged <21 years presenting with fever, and laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization with >2 organ system involvement
- No alternative plausible diagnosis
- Positive for current or recent COVID-19 infection by PCR, serology or antigen testing, OR exposure to a suspected or confirmed COVID-19 case within the past 4 weeks6
Although our patient was negative for COVID antigens and antibodies, it is possible that she may have cleared the infection by the time she developed KD symptoms. Furthermore, it is important to note that the novelty of the virus leads to uncertainties regarding the sensitivity and specificity of COVID-19 RT-PCR testing as well as antibody detection and interpretation. Recent studies, although limited by size and duration, have shown that these Kawasaki-like symptoms can develop weeks after exposure to COVID-19, and the majority of patients are not acutely infected with the virus at the time of presentation.7 Generally, antibodies are fairly reliable 4 weeks post-infection; however, some studies have shown that even in patients with positive RT-PCR results, 19% may be IgG seronegative at 4 weeks following exposure and up to 40% at 8 weeks.8
There is also significant overlap in symptomatology and pathophysiology of the two disease processes. Initial studies have provided strong evidence showing that COVID preferentially targets the protein angiotensin-converting-enzyme 2 (ACE2) on endothelial cells resulting in a systemic vasculitis similar to that seen in KD.9 This evidence is further supported by the fact that MIS-C generally presents with physical exam findings consistent with a diagnosis of atypical KD. Additionally, in both severe MIS-C and KD Shock Syndrome, there may be associated left heart systolic dysfunction and significant hypotension requiring vasoactive medications for hemodynamic support.10 KD has also been associated with multiple viruses, including the seasonal coronavirus strain linked to the common cold. With this knowledge and understanding, it may be more appropriate to describe this disease process as COVID-19 associated KD instead of trying to differentiate between MIS-C and KD.5 It is possible that MIS-C has been improperly classified as a separate entity from KD, when perhaps it is the same post-viral process we have been studying for years with Kawasaki Disease. The major difference in this case is that SARS-Cov-2 would be the first virus simultaneously linked to Kawasaki Disease and a global pandemic.
Considering these factors, in combination with the high prevalence of asymptomatic COVID carriers within the population, the presence of GI symptoms, and lab results that were consistent with an acute inflammatory syndrome, MIS-C remained high in the list of differential diagnoses.
Diagnosis
No single test provides a definitive diagnosis of KD. The diagnosis is based on clinical presentation and supported by characteristic laboratory abnormalities. According to the AHA, in order to make a diagnosis of Typical KD, a child must have a fever for at least 5 days AND have 4 of the 5 additional physical exam findings:
- Conjunctivitis (bilateral, painless, non-purulent)
- Mucocutaneous changes (cracked lips, strawberry tongue, erythema, pharyngitis)
- Polymorphous rash (diffuse, macular, may be scarlatiniform)
- Extremity changes (erythema, edema, desquamation)
- Lymphadenopathy (generally cervical and unilateral)
Patients with 5 or more days of fever and < 4 of the above findings should be considered for atypical KD with additional laboratory testing.11 If CRP >30 mg/L and or ESR >40 mm/hous; and if positive echocardiogram OR 3 or more of the below criteria are positive, patients should be treated as atypical Kawasaki.
- Anemia for age
- Platelets >450 x 106/microliter after the 7th day of fever
- Albumin level <30 g/L
- WBC count of >15 /microliter
- Urine >10 WBC /HPF
More severe disease, termed Kawasaki Shock Syndrome, often requires the use of vasopressors for hemodynamic support and is associated with increasingly high levels of CRP, procalcitonin, d-dimer, and IL-6.12 Elevations in NT-proBNP and troponin reflect cardiac inflammation and edema as opposed to myocardial ischemia.10
Management
Urgent echocardiogram is recommended for all cases of confirmed or suspected KD due to coronary artery manifestations. Although early treatment within 10 days of fever onset may prevent coronary artery pathology, recent studies have shown that up to 44% of patients will have coronary artery ectasia or aneurysms at the time of hospital presentation.3 Myocarditis and acute heart failure may also be seen.
Standard therapy for patients with KD involves IVIG (2 g/kg over 10-12 hours) and high dose aspirin (80-100 mg/kg/d divided into four separate doses). After fever resolution, the dose of aspirin is decreased to 3-5 mg/kg/d. IVIG can result in elevated ESR, thus changes in ESR should not be used as a gauge for disease progression or resolution. Steroids are indicated for IVIG resistant patients. Other agents have been seen in smaller trials to improve outcomes, notably Infliximab and Abciximab, however, more prospective studies are needed before they can be widely recommended.3
The clinician must be aware of the risk of recurrence, especially in children < 3 years old at the time of diagnosis. Repeat echocardiograms are indicated at 2 weeks and 6-8 weeks following hospital discharge. Patients should continue taking aspirin daily and may stop once the echocardiogram at the 6-8 week follow-up visit is negative for coronary artery abnormalities.
Case Conclusion
The patient received treatment with IVIG upon admission and had an uncomplicated inpatient course. Transthoracic echocardiogram showed normal cardiac structure and function with no evidence of proximal coronary artery aneurysm or ectasia. Initial urinalysis was consistent with UTI but repeat urinalysis the day following admission was negative. Stool PCR studies were positive for Enteropathogenic E. coli (EPEC). She continued to improve and was transferred to the floor after 2 days in the Pediatric ICU. Blood and urine cultures were also negative after 48 hours and antibiotics were discontinued. Parotid gland ultrasound showed cervical LAD. She was discharged on hospital day 4 with a diagnosis of Kawasaki Disease based on her negative COVID studies and relatively quick recovery. Her parents were instructed to continue giving her aspirin daily for continued prevention of complications related to coronary artery aneurysm.
She was seen in the pediatric cardiology clinic 2 weeks and 6 weeks following discharge from the hospital. Her mother noted persistent bilateral hand and foot swelling at the 2-week visit, but by 6 weeks, all symptoms had resolved. Repeat electrocardiograms and echocardiograms at those visits were reassuring with normal cardiac structure and function. Daily aspirin was discontinued, and she was scheduled to follow up in the clinic again in 6 months.
References
- Licciardi F, Pruccoli G, Denina M, et al. SARS-CoV-2–Induced Kawasaki-Like Hyperinflammatory Syndrome: A Novel COVID Phenotype in Children. Pediatrics. 2020;146(2):e20201711.
- Ramphul K, Gonzalez Mejias S. Kawasaki Disease: A Comprehensive Review. Arch Med Sci Atheroscler Dis. 2018;3:e41-e45.
- Seaton KK, Kharbadna A. Evidence-Based Management Of Kawasaki Disease In The Emergency Department. Pediatr Emerg Med Pract. 2015;12(1):1-20.
- Suowen X, Mingwu C, Jianping W. COVID-19 and Kawasaki Disease in Children. Pharmacol Res. 2020;159:104951.
- Calabri GB, Formigari R. Covid-19 and Kawasaki Disease: A Glimpse at the Past for a Predictable Future. Pediatr Cardiol. 2020;41(5):1075.
- Information for Healthcare Providers About Multisystem Inflammatory Syndrome in Children (MIS-C). 2021.
- Sandhaus H, et al. Association Between COVID-19 and Kawasaki Disease: Vigilance Required From Otolaryngologists. Otolaryngol Head Neck Surg. 2020;163(2):316-317.
- Long QX, Tang XJ, Shi QL, et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nature Medicine. 2020;26:1200-1204.
- Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J Clin Med. 2020;9(5):1417-1439.
- Belhadjer Z, Meot M, Bajolle F, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children (MIS-C) in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020;142:429-436.
- Son MB, Newburger JW. Kawasaki Disease. Pediatrics in Review. 2018;39(2):78-90.
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like Multisystem Inflammatory Syndrome in Children during the Covid-19 Pandemic in Paris, France: Prospective Observational Study. BMJ. 2020;369:m2094.