Left ventricular assist devices (LVADs) provide mechanical circulatory support in patients with advanced heart failure. As the number of heart failure patients grow, LVADs offer an opportunity for improved quality of life and survival vs. traditional cardiac transplant.
According to the Heart Failure Society of America, nearly 6.5 million Americans over the age of 20 have heart failure - with 960,000 new cases occurring annually. Heart failure directly accounts for around 8.5% of heart disease deaths in the U.S. and is a contributing factor in 36% of all cardiovascular disease deaths. It is expected that the number of heart failure patients by 2030 will grow to more than 8 million - and this will put a total cost of about $69.7 billion on health care.¹
Based on the Framingham Heart Study (a long-term, ongoing study), the mortality rate after a diagnosis of heart failure in the United States was around 10% at 30 days, 20-30% at 1 year, and 45-50% over 5 years.²
Initially meant to improve survival and bridge patients in the outpatient setting from their diagnosis of heart failure to donor transplantation, mechanical assistance of the left ventricle has evolved over the past few decades to the point it has become a destination therapy, especially for non-transplant candidates.³-⁴
Indications for LVAD placement⁵
- New York Heart Association Class IV Congestive Heart Failure - refractory to maximal medical therapy and conventional surgery
- Patients will present with discomfort at any level of physical activity. Symptoms present at rest.
- Ejection Fraction <25%
- Reduced functional capacity as measured by a maximal oxygen consumption VO2 <14 mg/kg/min
- Measure of the maximum amount of oxygen used during intense exercise
Contraindications for LVAD placement⁵
- Limited life expectancy
- Age >80 years old
- Active malignancy
- Severe comorbidities, including end-stage renal disease, severe liver disease, severe lung disease, severe vascular disease/arthritis, and neurologic disability
- Active severe bleeding/infection, intolerance to anticoagulation, chronic thrombocytopenia, refusal of blood products, and heparin-induced thrombocytopenia
- Anatomic disease such as congenital heart disease or a body mass index precluding implantation or rehabilitation
- Severe right heart failure, pulmonary hypertension, uncorrectable aortic insufficiency
- Evidence of ongoing substance use/dependency, inability to provide informed consent/adhere to medical regimen/maintain device, active mental illness or psychosocial instability
Components of an LVAD
Your typical LVAD patient may look something like this when you walk into the room:³
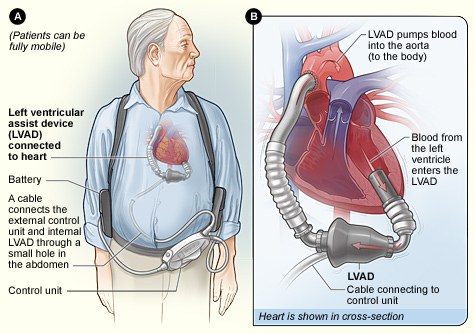
Source: National Heart Lung and Blood Institute (NIH)
Typical components of an LVAD include:
- Inflow cannula: pulls blood from the LV into the pump
- Pump: generates blood flow
- Outflow conduit: pushes blood into your ascending aorta
- Driveline: connects to an external controller
- External component: combination of your controller, monitors, power source, 2 batteries that monitors performance, displays battery life
All LVADs have four device parameters, with their normal values as follows:⁶
- Speed - set by the clinician - 8,000-10,000 RPM in the HeartMate II pictured above
- Flow - calculated - 4-6 L/min
- Power - energy applied to the pump to maintain flow - 4-6 Watts
- Pulsatility index - proportional to the left ventricle’s contribution to cardiac output - 1-10
Types
Currently, you’re most likely to see a continuous LVAD with the terrifying “pulseless” patient.
- Inflow cannula is continuously pulling blood from the LV into a single rotary pump operating at a fixed speed. Therefore, the flow, while continuous, is not constant and depends on preload.⁷
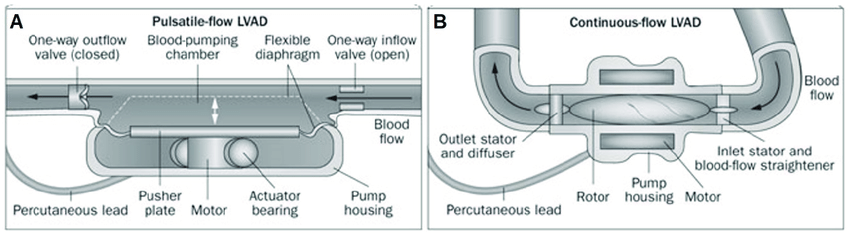
Pulsatile vs. Continuous Flow Key Takeaways
- First-generation LVADs were pulsatile in order to mimic the human heart; therefore, cardiac output was defined by the pulsatility of the device.⁸⁻⁹
- Pros: due to the pulsatile flow, hemodynamics were preserved
- Cons: placement required a median sternotomy, creation of a “pump pocket” that increases risk of infection and requires patients to have a large body habitus, a large-diameter percutaneous lead, an audible pump operation, with limited durability requiring device exchange
- Newer devices are continuous-flow (nonpulsatile).⁸⁻⁹
- Pros: smaller size, longer durability, higher energy efficiency, quieter, greater comfort, less risk of thrombotic events, reduced rates of infection, and less surgical trauma
- Cons: no physiologic pulse given that at higher pump speeds, the aortic valve is opening less and less (and perhaps not at all).10 One tradeoff is that blood pressure monitoring requires a manual blood pressure with Doppler or an arterial line
ED Assessment and Workup
- LVAD patients have a coordinator (think of them as a “point person”) who can help facilitate communication between the ED and the patient’s advanced heart failure team. They should be contacted even if the patient is not at the hospital where the LVAD was placed. Often the coordinator is aware prior to the patient coming in, but don’t make that assumption.
- Physical exam
- Stick with the basics! General appearance, pallor, capillary refill, urine output, mental status can give an idea of a potential shock state
- Auscultation: high-pitched continuous motor
- To obtain a blood pressure, inflate a manual blood pressure cuff to at least 120 mmHg systolic and slowly deflate while placing a doppler probe over the brachial or radial artery. The BP reading at the first return of sound approximates the mean arterial pressure
- If hemodynamically unstable, consider placing an arterial line for more precise monitoring
- Diagnostics
- Key labs: coagulation profile, ABGs for accurate oxygenation
- EKG
- Chest X-ray
- Bedside echo
- CT scan of the chest
- MRI contraindicated given pump magnet/metal
You do all of the above - non-LVAD complaint (complicated patient with simple problem) → normal ED treatment/workup. BUT, our complicated patient may be coming in with a complicated problem.
Key Emergent DDx for LVAD Patients
- Hemorrhage
- Suction event
- RV failure
- Arrhythmia
- Pump thrombosis
- Infection
- Stroke
RELAX. STAY CALM. CHECK YOUR OWN PULSE.
Remember, management of the unstable patient can be broken down into 3 components:⁴
- Classic ABCs
- This is an unstable patient, and you have managed unstable patients before, so start with the basics
- Any initial concern for cardiac arrest in the LVAD patient should lead to an evaluation of if the LVAD is running. Look for/auscultate for:
- i) Loud alarm
- ii) Depleted battery
- LVAD troubleshooting (if LVAD is off)
- Check the external components - open the LVAD bag and ensure all connections are secure.
- Check the battery charge
- Check that the controller is connected to the charged battery/power source
- Ensure the driveline is stable
- i) If none of the following are the cause or the LVAD cannot be restarted → proceed to ensuring adequate circulation and contact LVAD coordinator stat
- Ensure adequate circulation (if LVAD is on)
- Check cardiac rhythm with an ECG, physical exam for adequate perfusion, assess for evidence of thrombosis, drug use, hypoglycemia, bleed, and/or infection.
- i) Ventricular tachycardia or ventricular fibrillation -> immediate synchronized cardioversion or defibrillation
- If unresponsive despite the above efforts, no LVAD hum, MAP < 50 mmHg, PETCO2 < 20 mm Hg → CPR initiation
- i) Controversy to CPR initiation
- Risk of LVAD cannula dislodgement, anastomotic rupture (evidence is limited)
- Short periods of CPR appear to have favorable outcomes, so the benefit appears to outweigh the risk in cardiac arrest
- May give epinephrine consistent with ACLS
- i) Controversy to CPR initiation
- Check cardiac rhythm with an ECG, physical exam for adequate perfusion, assess for evidence of thrombosis, drug use, hypoglycemia, bleed, and/or infection.
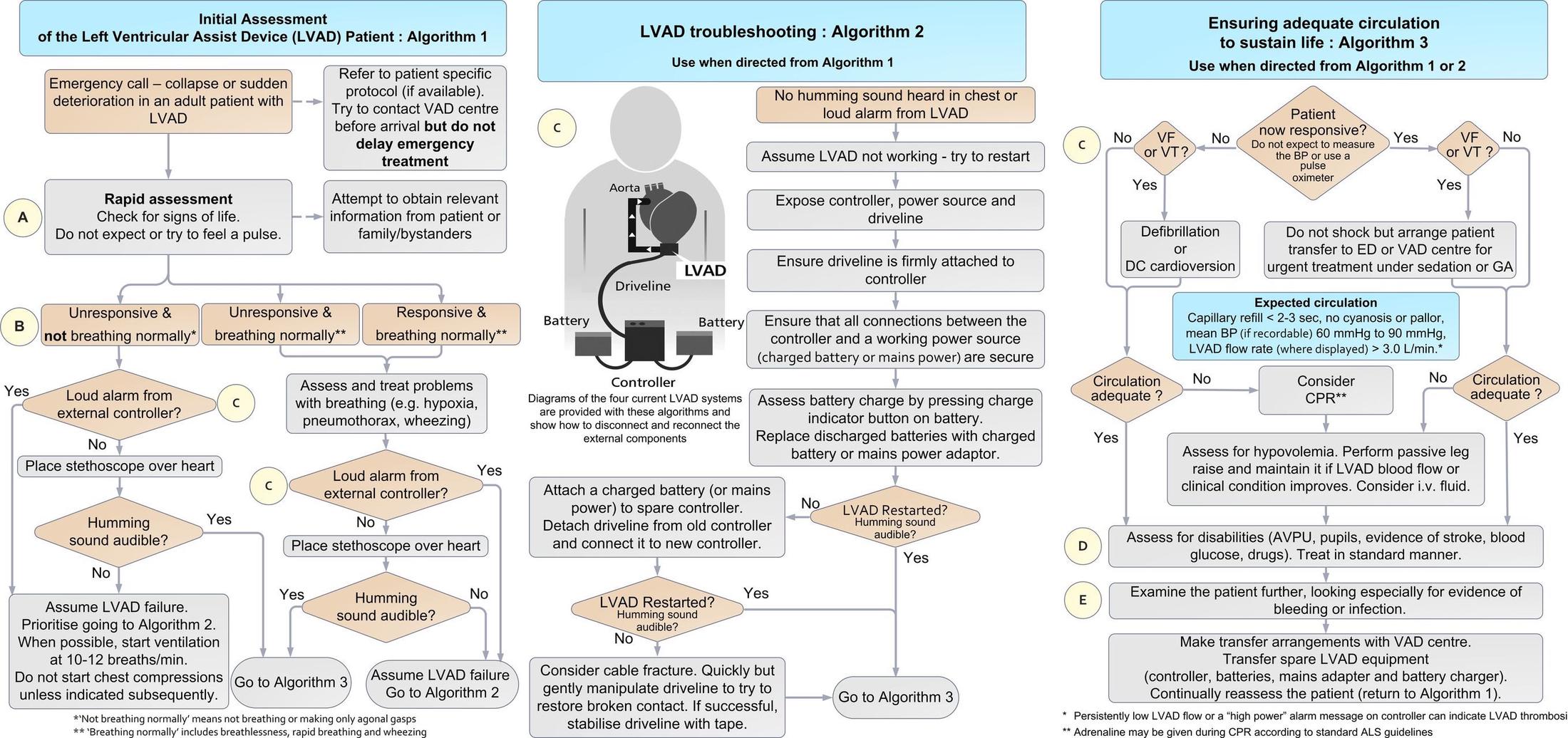
Breaking Down the Common LVAD Emergent DDx³
- Hemorrhage
- Reasons why patients bleed: on systemic anticoagulation, risk of acquired Von Willebrand’s disease associated with the shear force of the continuous flow LVAD, higher risks of GI bleeds and epistaxis from development of AV malformations due to reduced pulse pressure
- Considerations prior to treatment: thrombotic risk associated with LVAD if reversal of anticoagulation is done, alloimmunization from transfusion increasing the risk of organ rejection in the future, excessive volume. Discuss with the heart failure team.
- Suction event
- Underfilled LV or a distended septum (e.g., from RV failure/tamponade) → inflow cannula adheres to the LV wall → pump inflow suddenly decreases
- Patients can present with signs/symptoms of low cardiac output
- Most likely will need surgical exploration at an LVAD center, can consider increasing preload with IV fluids, but will need to evaluate for RV failure first
- Right heart failure
- Common post-op complication given that the LV is suddenly having an increased output, and now the RV must accommodate higher preload - leads to RV distension and possible failure
- Treatment considerations: inotropes, diuretics, pulmonary vasodilators, and possible RVAD placement
- Arrhythmia
- Given the continuous outflow LVADs provide, patients may be in lethal arrhythmias but continue to have full consciousness due to maintained cardiac output.
- Treatment considerations: ECG to detect the underlying abnormality, antiarrhythmic medications, shock/defibrillation
- Patients may have an underlying ICD due to this risk.
- Pump thrombosis
- Can form anywhere along the LVAD machinery - may be “typical” or secondary to denatured fibrin from the heat generated by the device.
- To be considered in: LVAD with a high power alarm, elevated markers of hemolysis on lab work, cardiogenic shock, or lack of hum on auscultation
- IV anticoagulation: if this fails consider pump-exchange
- Infection
- Historically the most common site is the driveline (often presents with cellulitis)
- Managed as a typical indwelling-device associated infection with IV antibiotics, will likely need to be in an LVAD center for definitive treatment.
- Stroke
- Peak incidence: early post-op and 9-12 months post-op
- Neither thrombolytics nor clot retrieval are recommended in an ischemic stroke, but there are cases where thrombolytics can be safely used in this population
- Intracerebral hemorrhage: consider anticoagulation reversal
Take-Home Points
- ABCs for any unstable LVAD patient run the same way you would any patient.
- Consider equipment failure and make sure to evaluate the key components with a combination of physical exam and diagnostics.
- The LVAD coordinator is your best friend and should be made aware of their patient’s ED visit as soon as possible.
- Keep in mind the common emergent differential diagnoses for LVAD patients as listed above, but remember that shock has the same etiologies in them as in any other patient.
References
- Heart Failure Society of America. Heart Failure Facts & Information. Accessed April 2023.
- Bytyçi I, Bajraktari G. Mortality in heart failure patients. Anatol J Cardiol. 2015;15(1):63-68.
- Hockstein MA. Continuous-flow left ventricular assist devices: Management in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(4):362-370.
- Bowles CT, Hards R, Wrightson N, et al. Algorithms to guide ambulance clinicians in the management of emergencies in patients with implanted rotary left ventricular assist devices. Emerg Med J. 2017;34(12):842-850.
- Han JJ, Acker MA, Atluri P. Left Ventricular Assist Devices. Circulation. 2018;138(24):2841-2851.
- Rezaie S. Left Ventricular Assist Device. REBEL EM blog, May 29, 2014. Available at: https://rebelem.com/left-ventricular-assist-device/.
- Rosenbaum AN, Antaki JF, Behfar A, Villavicencio MA, Stulak J, Kushwaha SS. Physiology of Continuous-Flow Left Ventricular Assist Device Therapy. Compr Physiol. 2021;12(1):2731-2767.
- Kato TS, Chokshi A, Singh P, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail. 2011;4(5):546-553.
- Miller LW, Pagani FD, Russell SD, et al., HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Purohit SN, Cornwell WK 3rd, Pal JD, Lindenfeld J, Ambardekar AV. Living Without a Pulse: The Vascular Implications of Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail. 2018;11(6):e004670.