This EMRA Critical Care Committee Deep Dive will review the current available literature on targeted temperature management for cardiac arrest.
Each year it is estimated that nearly 425,000 people experience EMS-assessed nontraumatic out-of-hospital cardiac arrest (OHCA) with survival to discharge in the range of 10% for all-comers.1 While a small fraction of these patients experience return of spontaneous circulation (ROSC) with good neurologic function, the large majority do not. It is in these patients that there is interest in reducing damage and preserving remaining neurological function by cooling their core temperature. Twenty years ago, a study published by Zeiner et al. showed a positive correlation between hyperthermia and unfavorable neurologic outcomes following cardiac arrest.2 Since then, studies have proposed numerous benefits of inducing hypothermia (known as targeted temperature management [TTM]), in patients who are post-cardiac arrest. Some of these benefits include decreasing cerebral metabolic demand, attenuating the production of oxygen free radicals, inhibition of deleterious inflammatory products (cytokines, interleukins, etc.), and restoration of normal intracellular signaling mechanisms and protein synthesis.3 Over the past two decades, TTM has been heavily researched as an intervention aimed at improving both survival and neurologic outcomes in various disease processes from traumatic brain injury (TBI) (POLAR),4 sepsis (CASS),5 and cardiac arrest. The focus of this article will be to review the landmark and most up-to-date literature on targeted temperature management in cardiac arrest.
Hyperthermia After Cardiac Arrest Is Associated With An Unfavorable Neurologic Outcome
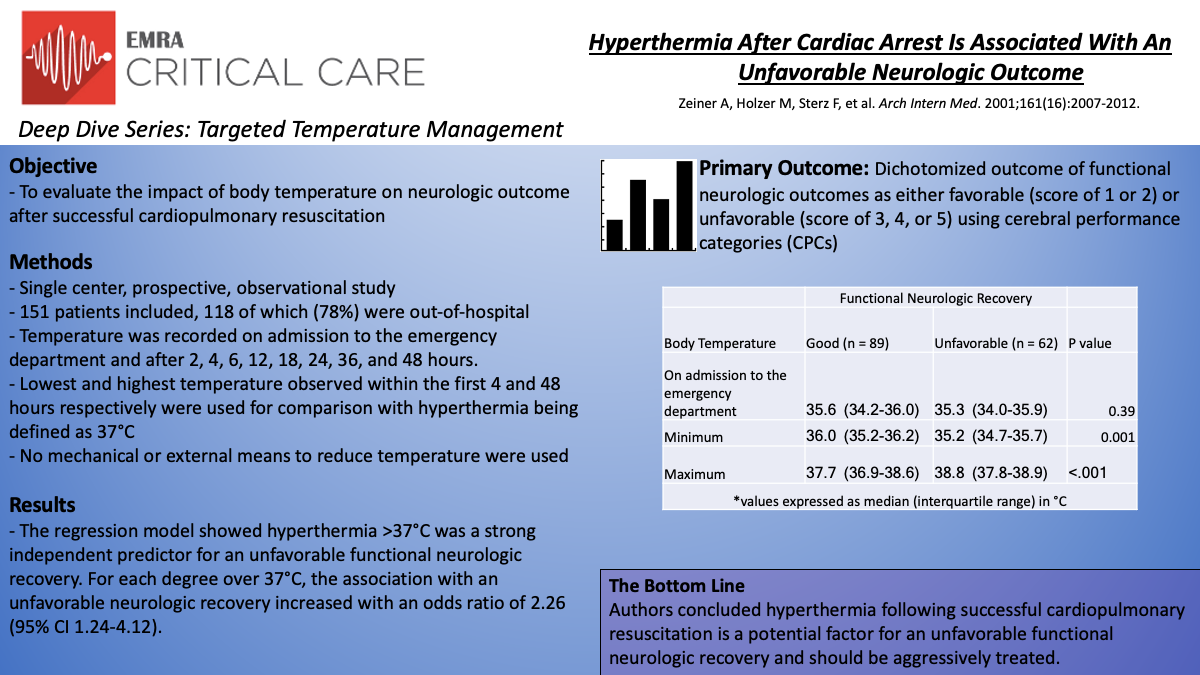
In this single-center, prospective, observational study, Zeiner et al. evaluated the impact of body temperature on neurologic outcome following witnessed cardiac arrest of presumed cardiac etiology. Temperature was recorded on admission to the emergency department and after 2, 4, 6, 12, 18, 24, 36, and 48 hours. The lowest and highest temperature observed within the first 4 and 48 hours respectively were used for comparison with hyperthermia being defined as 37°C. No mechanical or external means to reduce temperature were used. Functional neurologic outcomes were assessed using cerebral performance categories (CPCs) with the best-achieved score within 6 months used. A CPC score of 1 or 2 represents favorable neurologic recovery with scores of 3, 4, or 5 reflecting unfavorable recovery.
In all, 151 patients were enrolled and 118 (78%) of those were out-of-hospital cardiac arrests. Patients with favorable neurologic recovery showed a higher minimum temperature within 4 hours (median 36.0°C (IQR 35.2-36.2°C) versus 35.2°C (IQR 34.7-35.7°C) P=.001) and a lower peak temperature during the first 48 hours following ROSC (median 37.7°C (IQR 36.9-38.6°C) versus 38.3°C (IQR 37.8-38.9°C) P<.001). The regression model showed hyperthermia >37°C was a strong independent predictor for an unfavorable functional neurologic recovery. For each degree over 37°C, the association with an unfavorable neurologic recovery increased with an odds ratio of 2.26 (95% CI 1.24-4.12).
The authors concluded that hyperthermia during recovery from cardiac arrest worsened neurologic ischemic damage leading to less favorable outcomes. Studies investigating targeted temperature management, specifically hypothermia, as a way to mitigate these unfavorable outcomes followed.
Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia
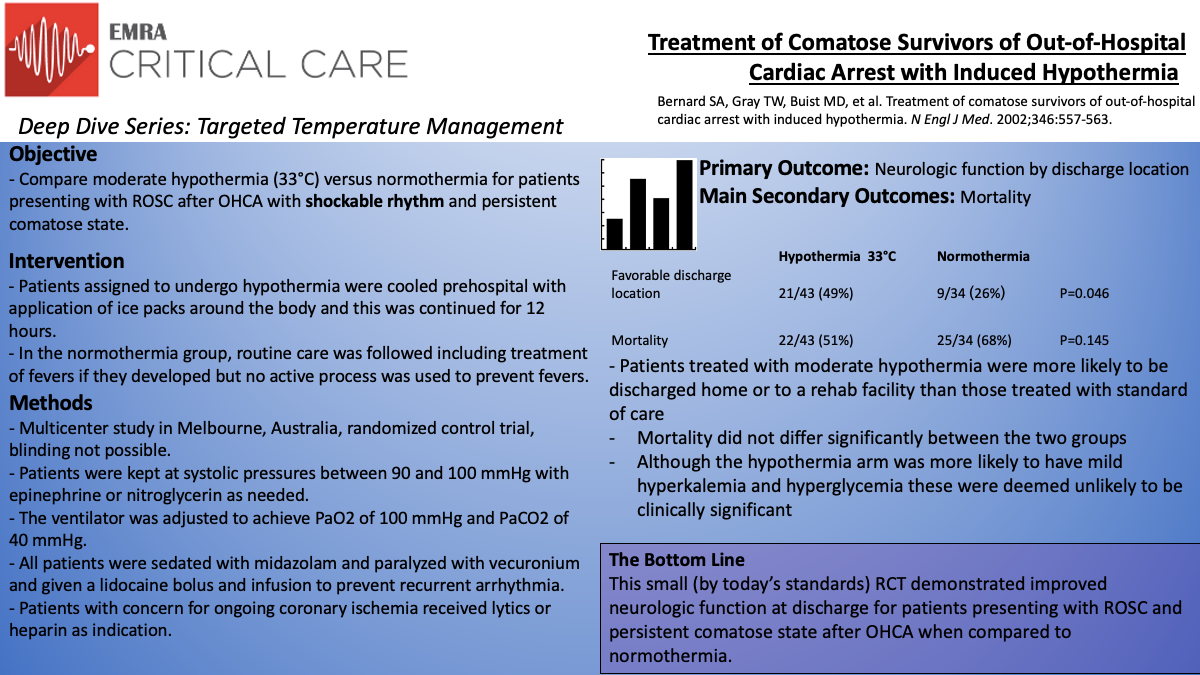
The Bernard paper6 was a randomized control trial out of Australia that built the foundation for post-cardiac arrest temperature management. The study was a prospective, randomized, multicenter study in Melbourne, Australia, from 1996 to 1999. Patients who had an out-of-hospital cardiac arrest with initial shockable rhythm with a persistent comatose state upon return of spontaneous circulation were considered. The cooling procedure began in the pre-hospital setting with cooling packs placed around the patient en-route to the hospital. Due to the nature of the intervention, blinding was not possible. Patients were assigned based on even and odd days of the month and the control group was standard of care without cooling. Cooling continued upon arrival at the hospital and for 12 hours total with the goal being 33°C as determined by a temperature foley catheter. Cooling was then stopped and if at the 18th hour the patient remained hypothermic they were rewarmed to normal body temperature and maintained there for 24 further hours.
In total, 77 patients were studied with 43 patients receiving hypothermia and 34 receiving standard of care. Excluded were those with other possible causes of coma (head trauma, overdose, stroke), cardiogenic shock (shock despite epinephrine infusion), and males younger than 18 and women younger than 50 (due to concern of possible pregnancy). Patients were cooled to the goal temperature over 2 hours. All patients received a bolus of vecuronium and were sedated with midazolam. Epinephrine infusions were used if systolic blood pressure was less than 90 mmHg and nitroglycerin was used if systolic blood pressure was greater than 100 mmHg. If the patient met clinical criteria for a STEMI they received thrombolytics and if they were found to have a NSTEMI they received heparin infusion. All patients received aspirin and bolus/infusion of lidocaine to prevent further dysrhythmia.
The primary outcome assessed was neurologic function at discharge. Patients were classified as having a favorable neurologic function if they were discharged to home or to a skilled nursing facility. Unfavorable neurologic outcomes included discharge to a long-term acute care facility, hospice, or if the patient died prior to discharge. One of the secondary outcomes was mortality. The findings of the study were that the patients treated with hypothermia were more likely to have a favorable neurologic outcome (49% versus 26%, p=0.046). Mortality did not differ significantly between the two groups.
Criticisms of this study include its relatively small size by similar more recent study's standards. Also, blinding was not feasible and therefore creates the opportunity for bias to be introduced. Interestingly, the hypothermia cohort received bystander CPR less often and it is hard to imagine how this would do anything but lead to worse outcomes for this group (which was the opposite of what was seen). The randomization process was not rigorous by conventional standards but analysis of the patients did not illuminate important differences between the two groups.
A generalized conclusion from this study was that induced hypothermia for 12 hours improved neurologic outcomes for patients presenting with out-of-hospital cardiac arrest. However, due to the small sample size, lack of true randomization, and other limitations further research was recommended to confirm these findings.
Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest (HACA Group)
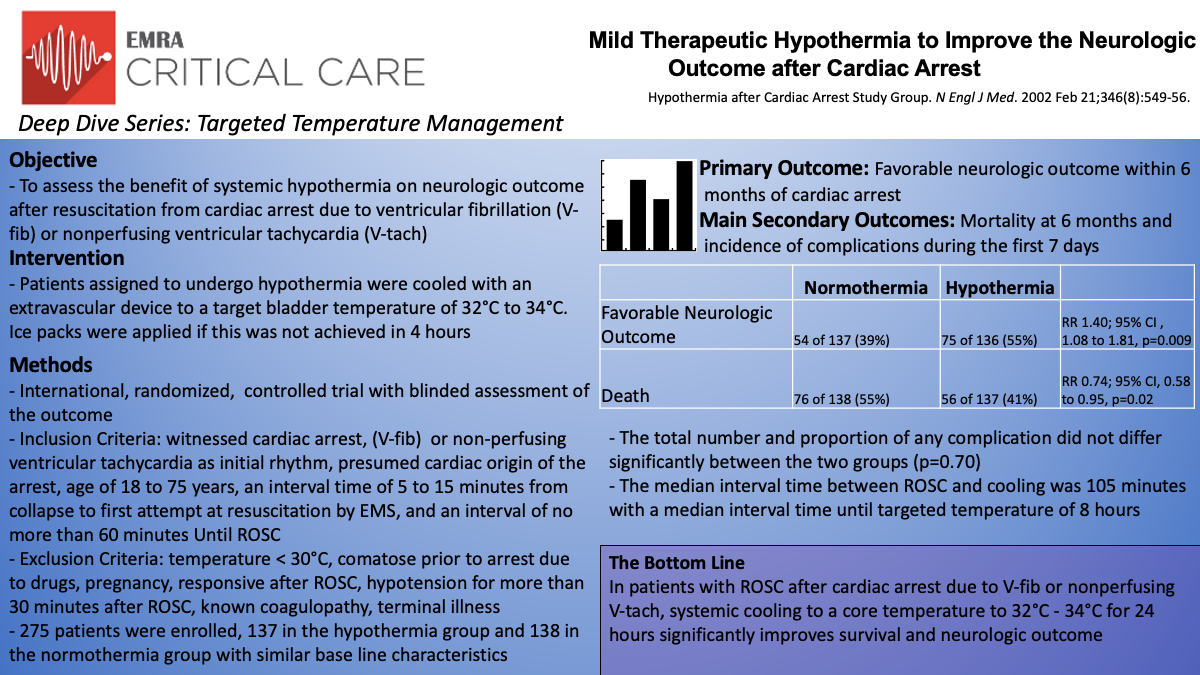
The Hypothermia After Cardiac Arrest (HACA) Study Group was one of the earlier studies investigating therapeutic hypothermia after cardiac arrest.7 In this multicenter, unblinded study, patients were randomized to either mild therapeutic hypothermia (32°C to 34°C) vs standard normothermia after resuscitation from cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia. The primary endpoint was favorable neurologic outcome within 6 months after cardiac arrest, assessed with the Pittsburgh Cerebral Performance Category (CPC). Mortality within 6 months and complications within seven days were also studied as secondary outcomes.
The multicenter trial included 275 patients (hypothermia group n=137, normothermia group n=138) across 9 centers in 5 European countries from 1996 to 2000. Inclusion criteria were age range of 18 to 75 with an estimated interval of 5-15 minutes from collapse to start of resuscitation, and a maximum interval of time to ROSC of 60 minutes. Exclusion criteria included comatose patient, hypothermic below 30°C, responsive after ROSC, coagulopathic, hypotensive (MAP 60 mm Hg for more than 30 minutes after ROSC), or hypoxic (arterial oxygen saturation, less than 85%). The goal for the hypothermia group was to be cooled to the target temperature (as measured via bladder-temperature probe) within 4 hours for 24 hours and then gradually warmed over a period of 8 hours.
This trial demonstrated 55% of the hypothermia group had a favorable neurological outcome (CPC 1 or 2) compared to 39% of the normothermia group (RR 1.40; 95% CI , 1.08 to 1.81, p=0.009). This trend continued with the secondary outcome of mortality at six months with 41% in the hypothermia group and 55% in the normothermia group (RR 0.74; 95% CI, 0.58 to 0.95, p=0.02). There was no statistical difference between the proportion or total number of adverse events between the two groups but sepsis was more likely to develop in the hypothermia group (17/135 in the hypothermia group vs 9/138 in the normothermia group). Hypothermia was initiated at a median interval of 105 minutes from restoration of spontaneous circulation and did take a median interval of 8 hours to achieve the targeted bladder temperature, double the goal of 4 hours.
The data from the HACA group does support the use of hypothermia in cardiac arrest. In this study, they demonstrated the hypothermia group had a 14% point lower rate of death at 6 months in cardiac arrest due to ventricular fibrillation or nonperfusing ventricular tachycardia (RR for the hypothermia group .74; 95% CI .58 to .95). However, the study does have some limitations. This study only included 8% of the patients assessed for eligibility, resulting in a small sample size. While there was no change in risk ratios after statistical correction, there were also slight differences in baseline characteristics between the two groups. These include history of diabetes mellitus, a history of coronary heart disease, and receipt of basic life support from a bystander, all being more common in the normothermia group. Two other key limitations in this study were that the normothermic group did become hyperthermic and both groups were at a higher risk of brain damage due to an interval time to first resuscitation starting at 5 minutes for inclusion criteria.
Targeted Temperature Management at 33C versus 36C after Cardiac Arrest: TTM
In this international, randomized trial, Nielson et al.8 investigated targeted temperature management at 33°C compared to 36°C in unconscious patients after out-of-hospital cardiac arrest of presumed cardiac cause. The primary outcome was all-cause mortality at the end of the trial. Secondary outcomes included rate of poor neurologic outcome or death at 180 days (assessed with Cerebral Performance Category [CPC] scale 3-5 and the modified Rankin scale 4-6), rate of adverse events, and analysis of pre-specified subgroups.
The trial included those patients 18 years or older who were unconscious (GCS <8 on admission) after out-of-hospital cardiac arrest of presumed cardiac etiology, irrespective of initial rhythm. All patients had >20 minutes of spontaneous circulation after resuscitation. Notable exclusion criteria included time from ROSC to screening >240 minutes, unwitnessed arrest with asystole as the initial rhythm, suspected or known ICH or stroke, and body temperature <30°C at randomization. A total of 950 patients were randomized, and 939 were evaluated for the primary and secondary analyses. After randomization, patients were cooled to a target temperature of 33°C or 36°C using intravascular or surface temperature management systems (76% surface and 24% intravascular in both groups). From 28-36 hours, the patients were gradually rewarmed to 37°C, followed by fever prevention (<37.5°C) until 72 hours post-arrest.
When assessed at 180 days, 54% of the 33°C group and 52% of the 36°C group had either died or had a poor neurologic outcome, as assessed by the CPC (RR; 1.02; 95% CI, 0.88-1.16; p=0.78). 52% of patients in both arms had died or had a poor neurologic outcome as assessed by the modified Rankin scale (RR 1.01; 95% CI, 0.89-1.14; p=0.87). At the end of the trial, a total of 50% of the patients in the 33°C group (235/473) had died, compared with 48% in the 36°C group (225/466) (HR of 33°C; 1.06; 95% CI, 0.89-1.28; p=0.51). Outcomes were consistent across subgroups, including initial rhythm, age, gender, time to ROSC, presence or absence of shock, and study site. Rate of adverse events was similar, with the exception of hypokalemia, which was more common in the 33°C group (19% vs 13% in 36C group).
The authors concluded that in unconscious patients after out-of-hospital cardiac arrest of presumed cardiac origin, targeted temperature management to 33°C conferred no significant benefit on mortality or neurologic outcome at 180 days when compared to a target temperature of 36°C.
Targeted Temperature Management for 48 vs 24 Hours and Neurological Outcome After Out-of-Hospital Cardiac Arrest
International resuscitation guidelines recommend TTM at 33-36°C for at least 24 hours, but the optimal duration is unclear. In this article, Kirkegaard et al.9 attempted to determine whether the use of TTM at 33°C for 48 hours resulted in better neurologic outcomes than the standard 24-hour TTM in unconscious patients with out-of-hospital cardiac arrest.
The study conducted was an international, blinded-outcome-assessor, parallel, pragmatic, multicenter, randomized clinical superiority trial. Three hundred fifty-five patients with out-of-hospital cardiac arrest were enrolled in the study group. Patients were randomized to TTM 33°C for 48 hours (176) or 24 hours (179) with both surface and invasive cooling methods allowed, followed by gradual rewarming of 0.5°C per hour until reaching 37°C. The primary outcome was a six-month neurologic outcome with a Cerebral Performance Categories score of 1 or 2 used to define a favorable outcome. Secondary outcomes included six-month mortality, including time to death, the occurrence of adverse events, and ICU resource use.
Of 355 patients who were randomized (mean age, 60 years; 295 [83%] men), 351 (99%) completed the trial. Of these patients, 69% in the 48-hour group had a favorable outcome at 6 months compared with 64% in the 24-hour group (difference, 4.9%; 95% CI, −5% to 14.8%; relative risk [RR], 1.08; 95% CI, 0.93-1.25; P = .33). Six-month mortality was 27% in the 48-hour group and 34% in the 24-hour group (difference, −6.5%; 95% CI, −16.1% to 3.1%; RR, 0.81; 95% CI, 0.59-1.11; P = .19). There was no significant difference in the time to mortality between the 48-hour group and the 24-hour group (hazard ratio, 0.79; 95% CI, 0.54-1.15; P = .22). Adverse events (new cerebral abnormalities, hypotension, cardiac arrhythmia, GI feeding intolerance, renal failure, infections, and bleeding) were more common in the 48-hour group (97%) than in the 24-hour group (91%) (difference, 5.6%; 95% CI, 0.6%-10.6%; RR, 1.06; 95% CI, 1.01-1.12; P = .04). The median length of intensive care unit stay was longer in the 48-hour group than in the 24-hour group (151 hours [IQR, 127-178 hours] vs 117 hours [IQR, 99-138 hours] respectively; P<.001), however there were no significant differences in hospital length of stay.
TTM at 33°C for 48 hours did not significantly improve the rate of favorable neurologic outcome or survival at six months when compared to TTM at 33°C for 24 hours in unconscious survivors from out-of-hospital cardiac arrest. Additionally, there were more adverse events related to longer TTM and no improvement in secondary outcomes. These findings may be limited due to power values; however, they currently support the current guideline for TTM at 33-36°C for 24 hours.
Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm
The HYPERION trial by Lascarrou et al.10 features a multicenter, randomized, controlled, assessor-blinded, superiority design across 11 university hospital ICUs and 14 community hospital ICUs in France. The study compared moderate hypothermia (33°C +/- 0.5°C) to targeted normothermia (37°C +/- 0.5°C) in comatose patients who had been resuscitated from cardiac arrest with nonshockable rhythms. Using the Cerebral Performance Category (CPC) scale, authors used a favorable neurologic outcome - defined as a CPC score of 1 or 2 at 90 days, as the primary outcome for the study. Authors analyzed mortality, duration of mechanical ventilation, hospital and ICU length of stay, infections and hematologic adverse events as secondary outcomes. Variables such as sedation, neuromuscular blockade and the management of adverse events were standardized for each of the two groups as part of the study protocol.
All patients 18 years or older, who had been resuscitated either out of hospital or in hospital from cardiac arrest with a nonshockable rhythm of any etiology were considered for inclusion in the study. Eligible patients also needed to be comatose (GCS 8 or lower) at ICU admission. Exclusion criteria included patients with more than 10 minutes from collapse to CPR, more than 60 minutes from CPR to ROSC, those requiring a continuous epinephrine or norepinephrine infusion of greater than 1µg/kg/min, patients with more than 300 minutes from time of cardiac arrest to screening, patients in moribund condition, Child-Pugh Class C cirrhotics, pregnant or breast-feeding, those under guardianship or current correctional facility inmates, patients who had previously been involved in a similar randomized controlled trial, uninsured patients and those whose next of kin refused study participation.
Authors used a web-based system that assigned patients randomly to each group on a 1:1 basis, and while staff may have been aware of group assignments, the psychologist responsible for assessing trial outcomes was blinded to group assignments. Between January 2014 and January 2018, 2723 patients were assessed for eligibility, 584 were randomized, of which 3 from the hypothermia group withdrew consent. Ultimately, 581 patients were included in the final study analysis (284 in the hypothermia group and 297 in the normothermia group). Cooling was stopped prematurely in 36 of the 284 hypothermia patients. The median age for both groups was 67 years old, 65% male in the hypothermia group 63% in the normothermia group and the study authors reported balanced clinical characteristics between the two groups.
Significant outcomes at 90 days included those with a CPC score of 1 or 2 - of which there were 29 in the hypothermia group (10.2%) and 17 in the normothermia group (5.7%), P=0.04. Of the hypothermia group, 231 patients (81.3%) were deceased at 90 days, compared with 247 in the normothermia group (83.2%). While there were differences in mortality rates, these results were not statistically significant (95% CI: -8.0 to 4.4). Similarly ICU mortality rates were 78.2% in the hypothermia group and 79.5% in the normothermia group (95% CI: 0.78-1.10). Survival to ICU discharge was 21.8% in the hypothermia group and 20.5% in the normothermia group (95% CI: 0.75-1.52) and survival to hospital discharge was 19.7% in the hypothermia group, compared with 16.8% in the normothermia group (95% CI: 0.81-1.74). Authors reported no significant differences in the duration of mechanical ventilation or the length of ICU stay among those who survived or those who died.
Authors ultimately concluded that comatose patients resuscitated from cardiac arrest with nonshockable rhythms, who survived at 90days were more likely to have a favorable neurologic outcome if moderate therapeutic hypothermia at 33°C had been used. However, the intervention did not have a greater benefit on survival at 90 days.
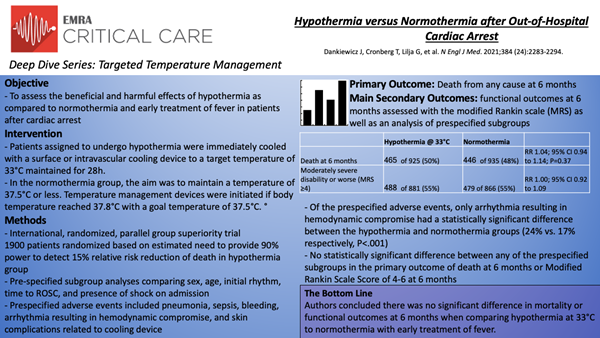
Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest: TTM2
In this international, randomized, parallel group, superiority trial, Dankiewicz et al.11 compared targeted hypothermia at 33°C to targeted normothermia at 36°C with early treatment of fever in patients with out-of-hospital cardiac arrest of presumed cardiac or unknown cause. The primary outcome was death from any cause at 6 months. Secondary outcomes included functional outcomes at 6 months assessed with the modified Rankin scale (MRS) as well as an analysis of prespecified subgroups (sex, age, initial cardiac rhythm, time to ROSC, and presence or absence of shock on admission).
Inclusion criteria were out-of-hospital cardiac arrest of cardiac or unknown cause, ROSC >20 minutes, unconscious as defined by the FOUR motor response <4 and no verbal response to pain. The main exclusion criteria were a time interval from ROSC to screening of >180 minutes, unwitnessed arrest with asystole as the initial rhythm, and limitations of care. 1900 patients were randomized and following those who withdrew consent (39) and lost to follow up (11), 1850 patients with similar baseline characteristics were evaluated for the primary outcome. Regarding the two treatment arms, in the hypothermia group the median time from the start of intervention until reaching 34°C was 3 hours and maintained at a target temperature of 33°C for 28 hours before rewarming. 53 of 930 patients in this arm were rewarmed early primarily due to cardiovascular instability and arrhythmias. A total of 882 of 930 (95%) in the hypothermia group and 428 of 931 (46%) in the normothermia group received cooling with a device.
At 6 months, 465 of 925 patients (50%) in the hypothermia had died as compared to 446 of 935 (48%) in the normothermia group (RR 1.04; 95% CI 0.94 to 1.14; P=0.37). Of 1747 patients in whom functional outcome was assessed, 488 of 881 (55%) in the hypothermia group had moderately severe disability or worse (MRS score ≧4) as compared to 479 of 866 (55%) in the normothermia group (RR 1.00; 95% CI 0.92 to 1.09). Similarly, the health-related quality of life as assessed using the EQ-5D-5L visual analogue scale was similar between groups (-0.8 points; 95% CI -3.6 to 2.0). Importantly, outcomes were consistent across all of the prespecified subgroups. Regarding adverse events, arrhythmias resulting in hemodynamic compromise were more common in the hypothermia group (24% vs. 17%; P<.001) with no significant differences between groups in rates of pneumonia, sepsis, bleeding, or skin complications due to the temperature management device.
Ultimately, in this randomized superiority trial comparing hypothermia with normothermia in patients with out-of-hospital cardiac arrest the authors concluded there was no significant difference in mortality or functional outcomes at 6 months with a higher reported incidence of arrhythmias resulting in hemodynamic compromise in the hypothermia group.
Conclusion
The most recent joint ILCOR and AHA advisory statement from 2015 recommends targeted temperature management for adults with out-of-hospital cardiac arrest at a constant temperature of 32-36°C for 24 hours for those with both an initially shockable rhythm (strong recommendation, low quality of evidence) and nonshockable rhythm (weak recommendation, very low quality of evidence).12 While the most recent literature points towards no significant difference between hypothermia and targeted normothermia, the past two decades of literature consistently associates fever at any time following cardiac arrest with worse outcomes. Given this, a protocolized approach to temperature management including pharmacologic and mechanical cooling with an aggressive avoidance of fever, proper sedation, and timely neuroprognostication remain staples of post cardiac arrest critical care.
References
- Go AS, et al. Heart Disease and Stroke Statistics – 2014 Update: A Report From the American Heart Association. Circulation. December 18, 2013.
- Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161(16):2007-2012.
- Koyfman, A., Long, B., Talavera, F., Setnik, G. and Peter, K., 2019. Targeted Temperature Management (Therapeutic Hypothermia): Practice Essentials, Overview, Pathophysiology. [online] Emedicine.medscape.com. Available at: <https://emedicine.medscape.com/article/812407-overview#a3> [Accessed 22 June 2021].
- Cooper DJ, Nichol AD, Bailey M, et al. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients With Severe Traumatic Brain Injury: The POLAR Randomized Clinical Trial. JAMA. 2018;320(21):2211-2220.
- Itenov TS, Johansen ME, Bestle M, et al. Induced hypothermia in patients with septic shock and respiratory failure (CASS): a randomised, controlled, open-label trial. Lancet Respir Med. 2018;6(3):183-192.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557-563. doi:10.1056/NEJMoa003289
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest [published correction appears in N Engl J Med 2002 May 30;346(22):1756]. N Engl J Med. 2002;346(8):549-556. doi:10.1056/NEJMoa012689
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206. doi:10.1056/NEJMoa1310519
- Kirkegaard H, Søreide E, de Haas I, et al. Targeted Temperature Management for 48 vs 24 Hours and Neurologic Outcome After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2017;318(4):341–350. doi:10.1001/jama.2017.8978
- Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N Engl J Med. 2019;381(24):2327-2337. doi:10.1056/NEJMoa1906661
- Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med. 2021;384(24):2283-2294. doi:10.1056/NEJMoa2100591
- Donnino MW, Andersen LW, Berg KM, et al. Temperature Management After Cardiac Arrest: An Advisory Statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2016;98:97-104.