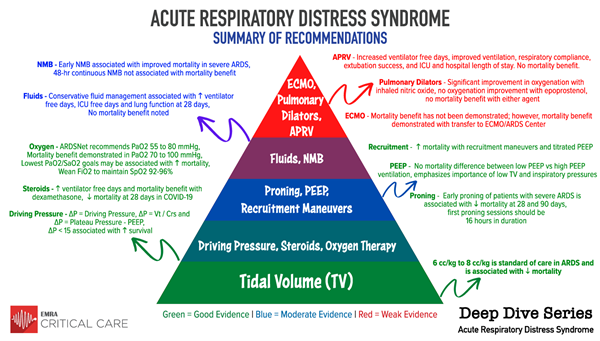
This EMRA Critical Care Committee Deep Dive will review the current best available literature on ARDS management and provide an insight on how to best treat ARDS patients in the emergency department.
Acute Respiratory Distress Syndrome (ARDS) is a life-threatening form of respiratory failure characterized by massive inflammation leading to pulmonary edema, decruitment of alveoli, and hypoxemia. Despite its incidence rate and lethality, there are limited direct therapeutic options available for its treatment.
Access the PowerPoint deck or read on to view the slides.
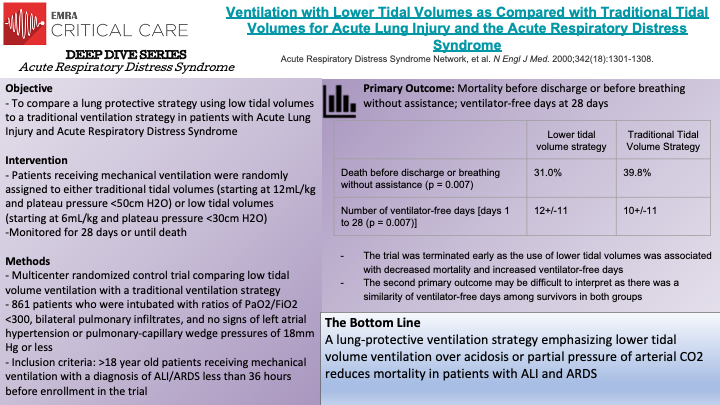
The ARDSnet trial, a multicenter randomized control trial, compared traditional ventilation (initial tidal volume of 12 mL/kg and plateau pressure <50 cm H2O) to lower tidal volume ventilation (initial tidal volume of 6 mL/kg and plateau pressure <30 cm H2O). The study enrolled 861 patients who were intubated and developed ratios of PaO2/FiO2 <300, bilateral pulmonary infiltrates, and no signs of left atrial hypertension or pulmonary-capillary wedge pressures of 18 mmHg or less (consistent with non-cardiogenic cause of pulmonary infiltrates). The trial was terminated early as the use of lower tidal volumes was associated with decreased mortality and increased ventilator-free days. The mortality rate of patients in the traditional ventilation group was 39.8% compared to 31.0% for patients receiving lower tidal volume ventilation. Additionally, the traditional ventilation group had an average of 12+/-11 ventilator-free days vs 10+/-11 for the lower tidal volume group. However, the authors note that this second primary outcome may be difficult to interpret as there was a similarity of ventilator-free days among survivors of both groups, potentially indicating that the results of this primary outcome were mainly driven by the reduced mortality seen in patients receiving lower tidal volume ventilation. Ultimately, ARDSnet demonstrated that lower tidal volume ventilation, placing a greater priority on lung-protective ventilation than concerns over acidosis or partial pressure of arterial CO2, reduces mortality in patients with acute lung injury and acute respiratory distress syndrome.
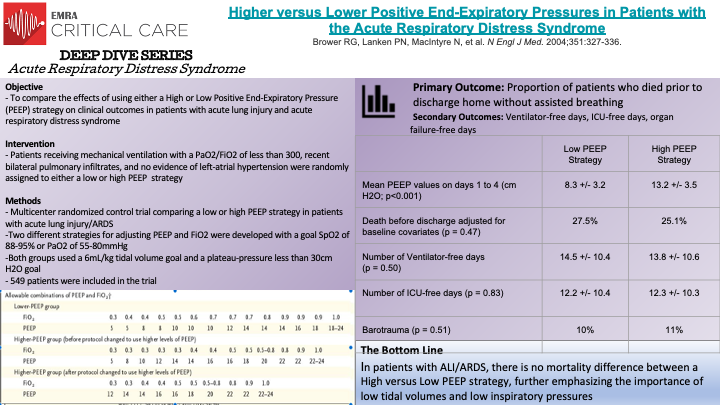
In this prospective, randomized-controlled trial, Bower et al randomly assigned individuals with acute lung injury and acute respiratory distress syndromes to receive mechanical ventilation with either a low or high PEEP strategy. 549 patients who were intubated with a PaO2 to FiO2 ratio of less than 300, recent bilateral pulmonary infiltrates, and no evidence of left atrial hypertension were eligible for randomization. All patients were treated with low tidal volume mechanical ventilation (6mL/kg) and with a goal plateau pressure of less than 30cm H2O. As demonstrated in the figure on the above slides, two different strategies were designed for adjusting PEEP and FiO2 with a goal SpO2 of 88-95% or a PaO2 of 55-80 mm Hg. The mean PEEP values on day 1 to day 4 were 8.3 +/- 3.2 cm of water in the low PEEP group versus 13.2 +/- 3.5 cm water in the high PEEP group (p<0.001).
The primary outcome was percent of patients who died prior to discharge. The unadjusted rate of death in the low PEEP group was 24.9%, compared to 27.5% in the high PEEP group (p=0.48). Of note, after 171 patients had been randomized, the high PEEP protocol was modified, and when adjusted for baseline covariates, the rate of death remained not significantly different; 27.5% mortality in the low PEEP group compared to 25.1% mortality in the high PEEP group (p=0.47). Secondary outcomes were also not significantly different. The number of ventilator free-days was 14.5 +/- 10.4 in the low PEEP group and 13.8 +/- 10.6 in the high PEEP group (p=0.50). The number of ICU-free days was 12.2 +/- 10.4 in the low PEEP group and 12.3 +/- 10.3 in the high PEEP group (p = 0.83). There was no significant difference in organ-failure-free days between the groups. It is important to note that both groups were treated with low tidal volume ventilation and low inspiratory pressures, which have been demonstrated to independently confer mortality benefit in patients with ARDS. The results of this study demonstrate that in patients receiving lower tidal volume ventilation and inspiratory pressures, there is no mortality difference between low and high PEEP strategies in patients with acute lung injury and acute respiratory distress syndrome.
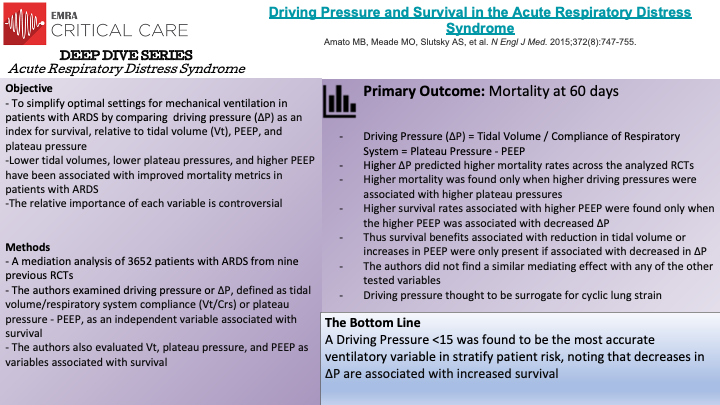
The Driving Pressure Trial analyzed several mechanical ventilation strategies to evaluate the effects of various lung-protective strategies on survival in ARDS patients. Specifically, the authors identified lower end-inspiratory (plateau) pressure, lower tidal volume, and higher positive end-expiratory pressure (PEEP) as strategies that have been associated with improved survival in patients with ARDS. However, previous studies had not completely identified the relative importance of each variable. In a mediation analysis of 3562 patients with ARDS from nine previously reported randomized trials, the authors hypothesized that Driving Pressure, defined as Tidal Volume divided by Compliance of the Respiratory System, or Plateau Pressure minus PEEP (effectively, ΔP = Vt / Crs and ΔP = Plateau Pressure - PEEP), would be more strongly associated with survival than either Vt or PEEP in mechanically ventilated patients. Notably, tidal volume was normalized to functional lung size instead of predicted lung size to more accurately reflect that in ARDS, the proportion of lung readily available for ventilation is decreased. The primary outcome of the Driving Pressure Trial was in-hospital survival at 60 days. The authors tested four variables (Vt, Plateau Pressure, PEEP, and ΔP or driving pressure), noting that ΔP was a dependent variable affected by changes in tidal volume and respiratory system compliance. Ultimately, higher ΔP predicted lower survival across the trials. Interestingly, higher mortality associated with higher ΔP was only found when higher plateau pressures were present. Higher levels of PEEP were found to be protective only if a higher PEEP was associated with a decrease in ΔP. Additionally, once lung protective strategies were employed, the authors did not find a survival benefit from further reducing plateau pressures below 30cm H2O nor tidal volume below 7mL/kg. Analysis of these studies thus demonstrated that reductions in tidal volume or increases in PEEP only improved survival when they were associated with decreased driving pressures. In recognizing that patients with ARDS have heterogenous lung areas when measuring compliance and that ΔP is likely a surrogate for lung strain, the authors of the Driving Pressure Trial demonstrated that in mechanically ventilated patients with ARDS ΔP was the variable most strongly associated with patient survival.
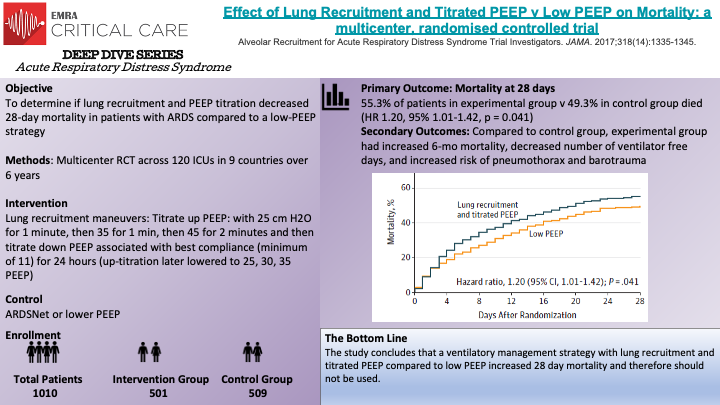
The physiologic rationale behind recruitment maneuvers is to increase PEEP in such a way to effectively open collapsed and de-recruited alveoli to both minimize atelectatic trauma of repeated opening and closing and create more functional lung space by re-recruiting collapsed alveoli. Prior to this study, non-randomized prospective studies found that recruitment maneuvers could result in opening nearly all alveoli. Two randomized trials and systematic reviews supported the idea that recruitment maneuvers may improve outcomes. This study was the largest, randomized controlled trial to examine the impact of recruitment maneuvers. Benefits of the study include the size, randomization, and standardization of protocols. Some critiques arouse with the recruitment protocol however. In the 5th year of the study after 2 cardiovascular arrests and data supporting worsened hemodynamics after and during recruitment the authors changed the protocol from 25, 35, 45 PEEP to 25, 30,35 PEEP however with this decrease there was no change in outcome in the original experimental group v the adjusted experimental group. Some reviewers pointed to the possibly the down titration as too aggressive and the larger culprit of the overall poorer outcomes rather than the recruitment maneuvers.
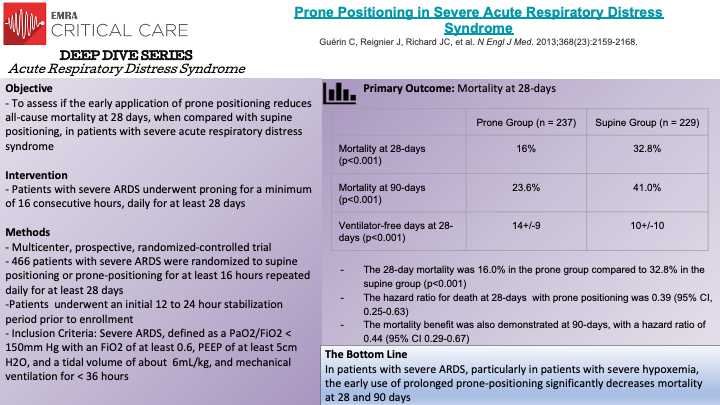
In this multicenter, prospective, randomized-controlled trial, Guerin et al. randomized 466 patient with severe ARDS (P/F < 150mm Hg with FiO2 of at least 0.6, PEEP of at least 5cm H2O, and Vt close to 6mL/kg) to either supine positioning or prone-positioning for at least 16 hours, to evaluate the effects of early proning in patients with severe ARDS. The primary outcome of the study was death of any cause at 28 days. Prior studies had demonstrated that oxygenation is improved when individuals are placed in the prone position by improving ventilation-perfusion mismatch; however, these physiological studies had not been applied to survival benefits in patients with ARDS. Patients being mechanically ventilated for ARDS for less than 36 hours, with a stabilization period of 12-24 hours, were randomly assigned to the control (supine) or to proning group. The patients in the proning group were placed in a prone position within one hour of randomization, and were required to remain prone for a minimum of 16 consecutive hours, and repeated daily for at least 28 days. Notably, low tidal volume ventilation (goal of 6mL/kg), plateau pressure goals of less than 30cm H2O, and a pH between 7.20 and 7.45 were used. The authors found that 16.0% of patients in the prone group died at 28 days, compared to 32.8% of patients in the control, with a statistically significant hazard ratio of 0.39. The significant mortality benefit was also demonstrated at 90-days; the unadjusted mortality at 90-days was 23.6% in the prone group versus 41.0% in the control, with a hazard ratio of 0.44. Ventilator-free days were also statistically higher in the prone group. Thus the PROSEVA Trial demonstrated that placing patients with severe ARDS in the prone-position significantly decreased 28-day and 90-day mortality. It is important to recognize that different institutions will have varying experience and comfort levels with placing patients in a prone position; proning patients can be technically difficult and requires several individuals working together to correctly, and safely, place a patient in the prone position.
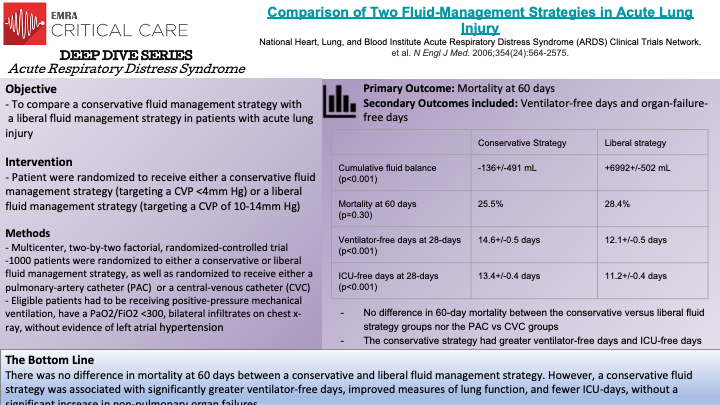
The FACTT Trial, a randomized-controlled trial, compared the effect of a conservative versus liberal fluid strategy on survival in patients with Acute Lung Injury/Acute Respiratory Distress Syndrome. In noting that acute lung injury causes increased capillary permeability, often leading to pulmonary edema, Wiedemann et al. sought to compare how two fluid strategies were related to death of any-cause at 60 days. A conservative fluid strategy generally restricts fluid intake and uses diuretics to increase urinary output to decrease pulmonary edema (“a dry lung is a happy lung”). Conversely, a liberal fluid strategy prioritizes fluid administration to maintain adequate cardiac output and non-pulmonary organ perfusion and function. 1000 patients were randomly assigned to either a conservative fluid strategy (defined by a target central venous pressure of <4mm Hg) versus a liberal fluid strategy (defined by a target central venous pressure of 10-14mm Hg). Patients in the conservative strategy had a seven-day cumulative fluid balance of –136+/- 491 mL. In the liberal strategy, patients had a seven-day cumulative fluid balance of 6992+/-502 mL. Ultimately, in-hospital death at 60 days was not statistically significant between the two groups; the conservative group had a 25.5% mortality rate at 60 days compared to a 28.4% mortality rate at 60 days in the liberal group. However, the conservative group did have a statistically significant increase in ventilator-free days and ICU-free days, as well as improved lung function at 28-days. Thus, in patients with ALI/ARDS, a conservative fluid strategy was demonstrated to improve lung function, decrease ventilator-free days and reduce ICU stays when compared to a liberal fluid strategy.
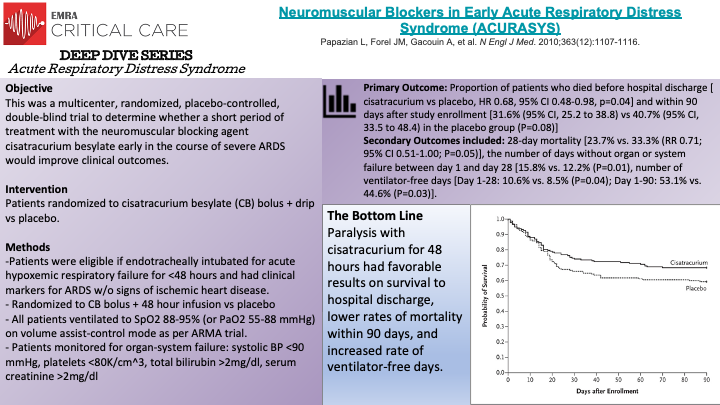
The ACURASYS Trial was a multicenter, double-blind, randomized controlled trial evaluating the effect of neuromuscular blockade on survival in 340 mechanically ventilated patients with acute (defined as within 48 hours) onset of severe ARDS. Patients were randomly assigned to receive either 48-hours of continuous cisatracurium or placebo. The primary outcome was the proportion of patients who died before hospital discharge or within 90 days of enrollment. The crude mortality rate at 90-days was not significantly different between groups. However, when adjusted for baseline PaO2/FiO2, plateau pressure, and Simplified Acute Physiology II score the trial found a significant hazard ratio of 0.68 for death at 90-days in the intervention group compared to the control. The intervention group also had significantly greater ventilator-free days at 28 and 90-days, days free from organ failure at 28-days, and lower rates of pneumothorax when compared to the control. Interestingly, the rates of ICE-acquired paresis or weakness was not significantly different between the groups. Thus, the ACURASYS Trial was able to demonstrate that early administration of neuromuscular blockade in patients with severe ARDS may improve mortality and increase the number of ventilator-free days.
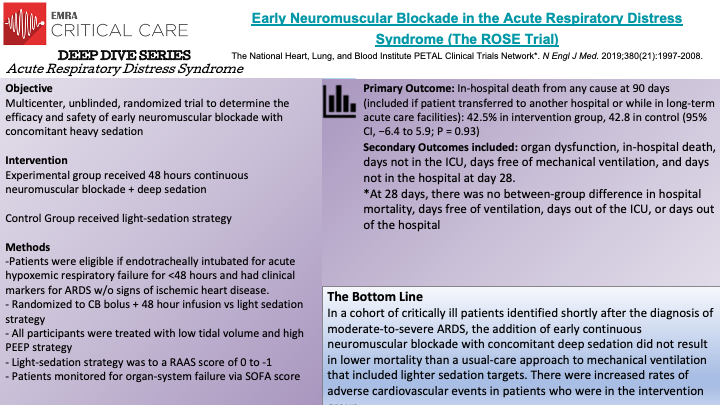
The ROSE Trial, a multicenter randomized trial, evaluated the benefits of early continuous neuromuscular blockade in mechanically ventilated patients with moderate-to-severe ARDS. 1006 patients with moderate-to-severe ARDS (defined by PaO2/FiO2 ratio of less than 150mm Hg, a PEEP of 8cm H2O or greater, bilateral pulmonary opacities on radiography, and noncardiogenic respiratory failure) were randomized to either a 48-hour continuous cisatracurium infusion with concomitant deep sedation versus usual-care (no routine neuromuscular blockade with lighter sedation goals). The ROSE trial was designed in an effort to maintain consistency with the ACURASYS trial, which did demonstrate lower mortality with 48-hour continuous infusions of a neuromuscular blockade, with the notable exception of lighter sedation goals in the control or usual-care group. The primary endpoint was in-hospital death, from any cause, at 90 days. The trial was terminated at the second interim data analysis for futility. The mortality rate at 90-days in the intervention group was 42.5% compared to 42.8% for the usual-care group, demonstrating no significant difference in 90-day mortality. Moreover, at 28-days there were no between group differences in mortality, ventilator-free days, ICU-free days, nor hospital-free days. The intervention group will have significantly more serious cardiovascular events when compared to the usual-care group. Ultimately, the ROSE Trial was unable to replicate the mortality benefit of early 48-hour continuous neuromuscular blockade in patients with moderate-to-severe ARDS.
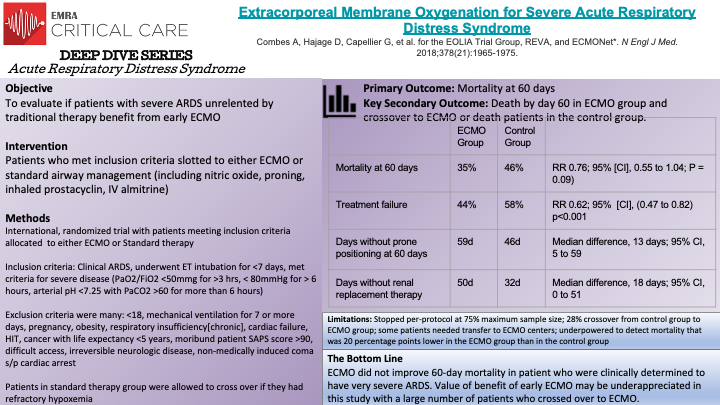
This is a multi-center, non-blinded, randomized-controlled, crossover trial conducted by Combes, A., et al to determine the effect of early ECMO in patients with severe ARDS. Inclusion criteria for this study were patients who med the AECC (American-Europan Consensus Conference) definition of ARDS, they had undergone endotracheal intubation for <7 days, and met disease severity as outline by study authors ( P/F <50mmg for >3 hrs, < 80mmHg for > 6 hours, arterial pH <7.25 with PaCO2 >60). Patients meeting criteria were slotted to either ECMO or standard airway management (including proning, nitric oxide, inhaled prostacyclin, IV almitrine). Patients who were allowed to crossover from the control (standard therapy) group if they had refractory hypoxemia (<80% SaO2 for >6hrs). There were a number of secondary outcomes including treatment failure (crossover to ECMO or death and per-treatment analysis. 240 patients were included in this study: 124 allotted to ECMO (3 did not receive ECMO treatment ) and 125 to control group (35 crossed over to ECMO group). The primary outcome was mortality at 60 days: 35% mortality in ECMO group and 46% in control group (p=0.09). At 60 days, those allotted to ECMO had significantly more days than those in control group free from renal failure or requiring RRT. Conclusion: In patients with very severe ARDS, 60 day mortality was not significantly lower with ECMO than with a strategy of conventional mechanical ventilation that included ECMO as rescue therapy.
Limitations for this study: stopped per-protocol at 75% maximum sample size, 28% crossover from control group to ECMO group, inclusion of patients presenting to non-ECMO centers requiring transport to ECMO center if randomized to ECMO group.
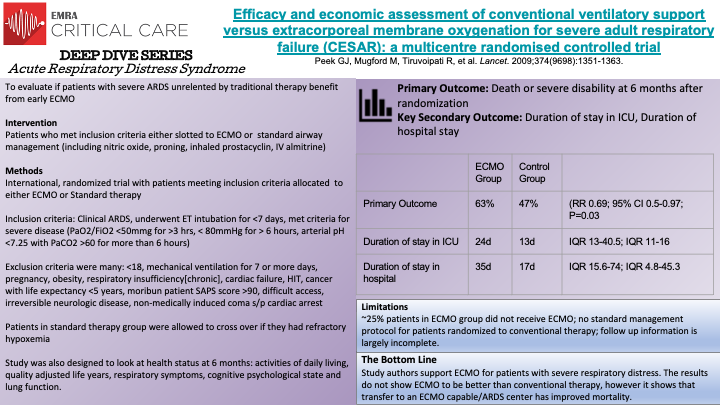
This is a multicenter, non-blinded,randomized control trial evaluating whether ECMO is a safe, efficacious and cost-effective treatment for severe adult respiratory failure when compared to conventional management. Though study authors support use of ECMO, the data did not support that it was any better in reducing mortality when compared to conventional therapy. Limitations included: ~25% patients in ECMO group did not receive ECMO; no standard management protocol for patients randomized to conventional therapy; follow up information is largely incomplete.
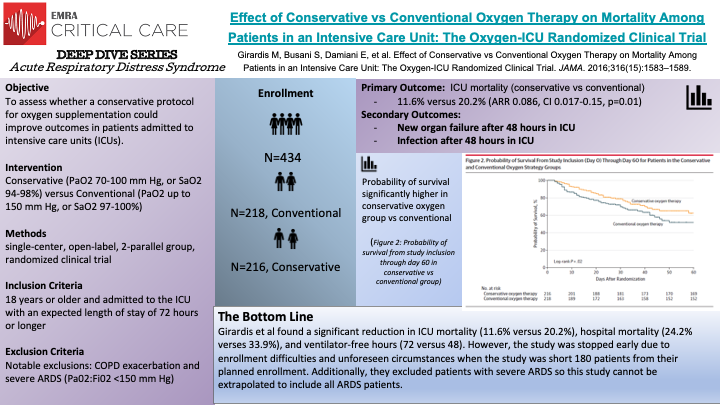
In this single center, randomized controlled trial, Girardis et al randomized 434 patients to receive conventional oxygen therapy (218) versus conservative oxygen therapy (216). The conventional oxygen therapy group allowed PaO2 values up to 150 mm Hg or SpO2 values between 97% and 100%, while the conservative therapy group maintained PaO2 between 70 and 100 mm Hg or SpO2 between 94% and 98%. The primary outcome of the study was ICU mortality. Supplementation of oxygen for hypoxemia is an integral component of medicine as a whole and studies examining the negative effects of unbridled oxygen supplementation demonstrated potential for harm such as increased long term mortality in the PROXI trial and potentially increased early myocardial injury in the AVOID trial. All patients 18 years and older and admitted to the ICU with an expected length of stay of 72 hours or longer were considered for inclusion in this study. Patients were randomly assigned to the control group and received FiO2 of at least 0.4, allowing PaO2 values up to 150 mm Hg and SpO2 between 97% and 100% or to the protocol group with the lowest possible oxygen supplementation to maintain PaO2 between 70 and 100 mm Hg or SpO2 values between 94% and 98%. At least one arterial blood gas sample was collected per day for each patient. The authors found a significant reduction in ICU mortality (11.6% versus 20.2%), hospital mortality (24.2% verses 33.9%), and ventilator-free hours (72 versus 48). Several caveats to the study are warranted, the study was stopped early resulting in decreased enrollment, approximately 180 patients short of the goal. Additionally, this was a single center trial and from the data, it appears the conventional oxygen supplementation group was sicker at baseline which may have exaggerated the effect on mortality. Lastly, the study excluded patients with severe ARDS, which is an important caveat when we are considering the best oxygen supplementation strategies for ARDS.
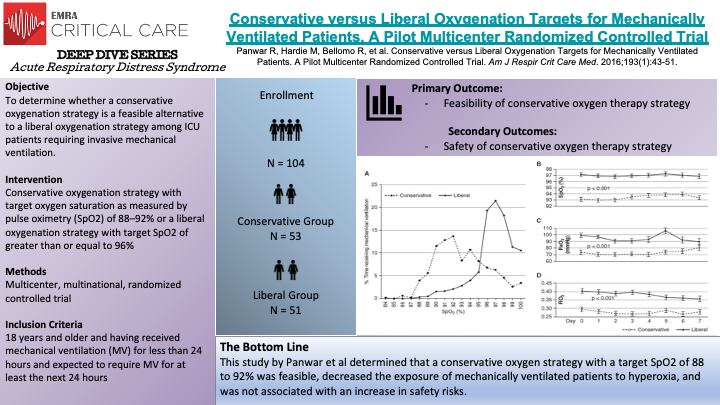
In this multicenter, multinational, randomized controlled trial, Panwar et al randomized 103 patients were randomly assigned to either conservative oxygenation strategy or to liberal oxygenation strategy. The objective of the study was to determine if a conservative oxygenation strategy is a feasible alternative to liberal oxygenation in ICU patients requiring invasive mechanical ventilation. The authors also sought to obtain safety data on such a strategy. The primary endpoints were mean area under the curve (AUC) for SpO2, SaO2, paO2, and FiO2 on days 0 through 7. The conservative oxygenation group had their FiO2 titrated to a target of 88 to 92% SpO2 while the liberal oxygenation group had their SpO2 target of greater than or equal to 96%. In total, 53 patients were assigned to the conservative oxygenation group and 51 patients assigned to the liberal oxygenation group. The authors found that the participants spent majority of the time within their targeted SpO2 range in each group. The mean AUC and 95% confidence intervals for SpO2, SaO2, PaO2, and FiO2 were significantly lower in the conservative group compared with the liberal group. They also found that participants spent more time at an FiO2 of 0.21 than those in the liberal group. The authors found the liberal group had lower vasopressor dose requirements, however no significant difference was found between groups in measures of organ dysfunction, vasoporessor duration and hospital length of stay. The strengths of this study include multicenter multinational randomized controlled design, protocol adherence, clear separation between groups, and detailed longitudinal data. The weaknesses of the study primarily are lack of blinding, not being powered to test superiority of the two strategies, inattention to other potentially important clinical endpoints, allowing clinicans to alter oxygenation targets if clinically indicated, among others.
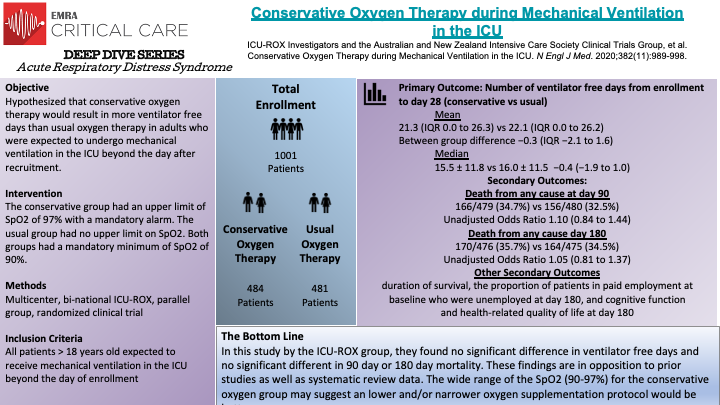
In this binational, multicenter, randomized clinical trial, the ICU-ROX study group and the Australian and New Zealand Intensive Care Society Clinical Trials group randomized 1,000 total patients to a conservative oxygen therapy group or usual oxygen therapy group. Due to patient consent withdrawal, 965 patents were left for the intention-to-treat analysis, 484 assigned to the conservative-oxygen group and 481 to the usual oxygen group. The primary outcome of this study was number of ventilator-free days from randomization until day 28. In the conservative oxygen group, the FiO2 was decreased to 0.21 and supplemental oxygen discontinued in patients who had been extubated if the SpO2 was above acceptable lower limit. The conservative group had a mandated alarm set to sound when SpO2 was 97% or greater to minimize exposure to SpO2 greater than 97%. In the usual oxygen group, no specific measures limiting the FiO2 or the SpO2 were made and the use of an upper alarm limit for SpO2 was prohibited as well. The use of FiO2 less than 0.3 was discouraged in this group as well. The authors found no significant difference in the number of ventilator-free days between the conservative and usual oxygen therapy groups. There was also no evidence of significant between-group differences in 90-day mortality, 180-day mortality, or survival. They found that patients spent significantly more time receiving FiO2 of 0.21 and significantly less time with and SpO2 greater than 97%. The limitations of this study include lack of blinding and that findings may not apply to less sick patients, among others. The oxygen supplementation protocol in this study is vaguer when compared to the Oxygen-ICU trial. These authors report that the oxygen exposure in the usual-care group in this trial and the control group were similar to the Oxygen-ICU trial, however, they don't comment on any similarities between the treatment groups which would be more interesting to compare.
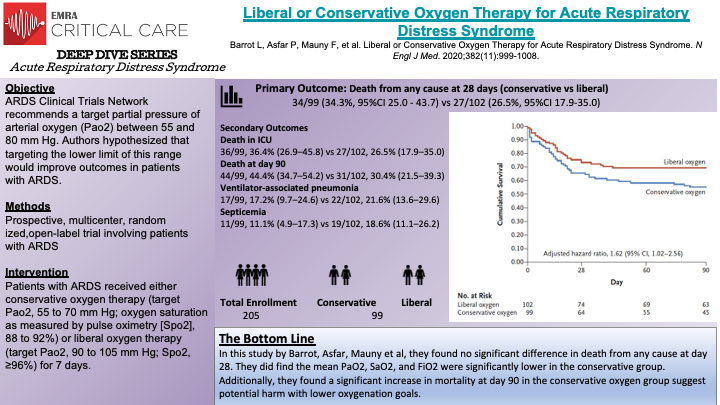
In this prospective, multicenter, randomized, open-label trial, Barrot et al randomized 205 patients to the liberal oxygen group or the conservative oxygen group. The trial was stopped early due to safety concerns of potential increased risk of serious adverse events and futility from the independent safety board monitoring the study before the goal of 850 patients were enrolled. The primary outcome of the study was death from any cause at 28 days after randomization. In the liberal oxygen group PaO2 target was between 90 and 105 mm Hg and the conservative oxygen group PaO2 target was between 55 and 70 mm Hg, both of which were maintained over the first 7 days of invasive mechanical ventilation or until extubation. Volume assist-control mode of ventilation was recommended, with a tidal volume of 6 mL per kg of predicted body weight. PEEP was adjusted according to PaO2:FiO2 ratio; between 200 and 300 mm Hg, PEEP was between 5 and 10, less than 200 mm Hg, PEEP was sat at maximal value to reach plateau pressure of 28 to 30. The authors found that the mean PaO2, SaO2, and FiO2 were significantly lower in the conservative group versus the liberal group. At day 28, mortality was not significantly different between the two groups, 34.3% vs 26.5%. At day 90, mortality was significantly higher in the conservative oxygen group, 44.4% versus 30.4%. Notably, the difference in FiO2 between the two groups in this study was larger than in the OXYGEN-ICU and CLOSE trials. The strengths of this trial include prospective multicenter design, well balanced groups, and how specific the protocol was for both groups.
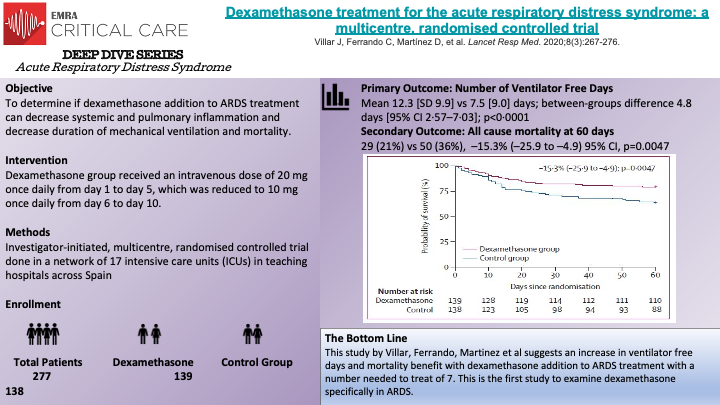
In this multicenter, randomized controlled trial, Villar, J. et al randomized 277 patients to the dexamethasone treatment group or to the control group. The trial was stopped early by the data safety monitoring board due to low enrollment rate after enrolling more than 88% of the planned sample size (277/314). The primary outcome of the study was number of ventilator-free days at 28 days, defined as days alive and free from mechanical ventilation. The secondary outcome was all-cause mortality 60 days after randomization. Patients assigned to the dexamethasone group received the first dose immediately after being randomized and no later than 30 hours after ARDS onset. The dexamethasone group received 20 mg once daily from day 1 to 5, which was then reduced to 10 mg once daily from day 6 to 10. Patients in the control group were designated to receive continued routine intensive care (conventional care). Low protective ventilation was followed in both groups including tidal volume of 4 to 8 mL/kg of predicted body weight, plateau pressures less than 30 cm H2O, respiratory rate to maintain PaCO2 between 35 and 50 mm Hg, and with PEEP and FiO2 combinations according to the ARDSNet Protocol table. The authors found that patients in the dexamethasone group had a greater mean number of ventilator free days (12.3 [SD of 9.9] vs 7.5 [SD of 9.0], p <0.0001). For the secondary outcome, the authors found that at 60 days after randomization, 29 patients (21%) in the dexamethasone group versus 50 patients (36%) in the control group had died (p = 0.0047). Several strengths of the study are noted including the largest RCT done investigating corticosteroids in moderate-to-severe ARDS, specific criteria for enrollment, criteria for time of enrollment and reassessment of patients for appropriateness for inclusion at 24 hours post enrollment.
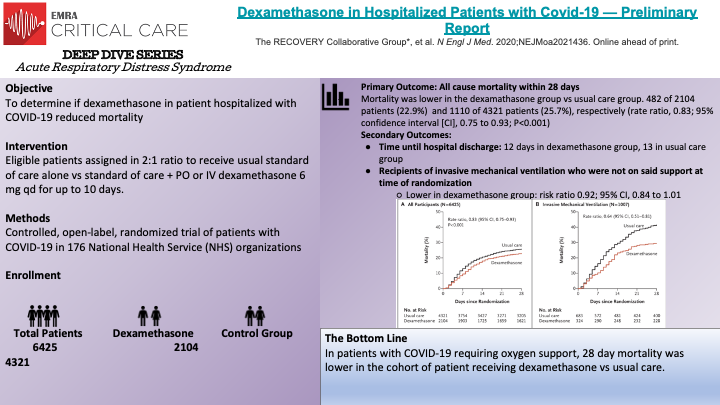
Randomized, controlled, open-label study evaluated the role of dexamethasone in hospitalized patient who had COVID-19. Patients were slotted to either usual care vs usual care + dexamethasone. The open label nature of this study means that both the study participants and researchers knew what medication they were receiving. Inclusion criteria included >18 years of age, clinically significant COVID infection requiring hospitalization, pregnant patients were eligible. Exclusion criteria include patients being discharged home. Major take away: Dexamethasone reduced 28-day mortality in patients requiring oxygen support who have COVID -19. Increased rates of harm may be associated with dexamethasone administration in COVID-19 patients who DO NOT require oxygen support. Strengths: large sample size, >95% follow up, >95% received study drug if alloted to Limitations: open label, preliminary report
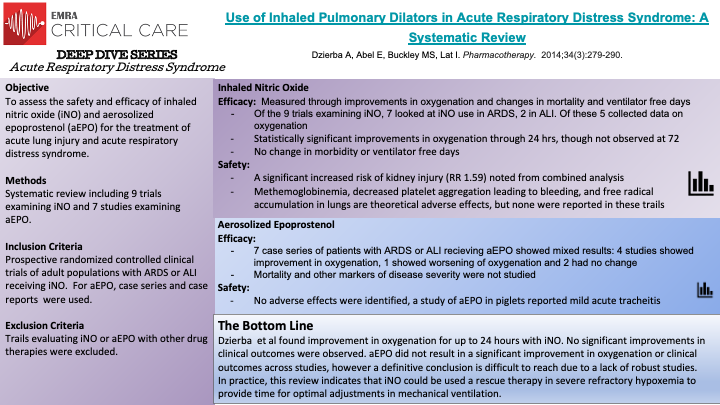
In this systematic review, 9 prospective randomized controlled clinical trials of adult patients with ARDS or ALI receiving iNO and 7 studies examining the effect of aEPO were reviewed to assess the safety and efficacy of aEPO and iNO. The review included all prospective randomized controlled trials of adult patients with either ARDS or ALI that received iNO. No randomized controlled trials of aEPO existed at the time of this review, so the authors included any case series or case reports examining the efficacy and safety of aEPO. Regarding iNO, a statistically significant improvement in oxygenation was noted among the trials, two of which examined oxygenation at 24 hours. This improvement in oxygenation was not observed at 72 hours in the one RCT with this extended time point. Despite this improvement in oxygenation, there was no significant improvement in clinical outcomes (mortality, ventilator free days) in patients receiving iNO. Additionally, there was a significant increased risk of kidney injury in patients receiving iNO.
Regarding aEPO, there was no robust improvement in oxygenation or clinical outcomes observed across the reports included in this review. Additionally no adverse outcomes were noted.
As with any systematic review, the quality of the review depends on the quality of the studies included. A true lack of robust trials of aEPO limit any conclusions that could be drawn regarding safety and efficacy of the treatment, though based on this review the results do not appear promising. With regard to the iNO trials, the number of patients involved was limited thus decreasing the power, even in a combined analysis to detect changes in clinical outcomes or morbidity. Based on the data from this review, iNO seems to be best used clinically as a rescue therapy for severe refractory hypoxemia until more data is avalible.
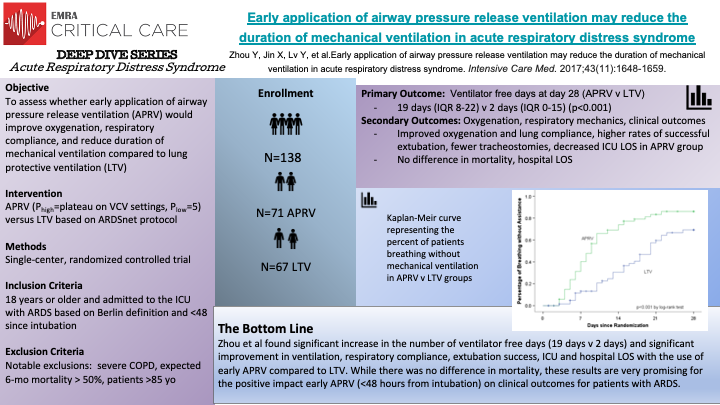
This single-centered RCT of 138 patients is the largest trial examining APRV vs LTV as the ventilation strategy for intubated patients with ARDS. The early application of APRV was associated with better oxygenation, less sedation, fewer days on mechanical ventilation, and shorter ICU stays compared to LTV. However, there was no difference in mortality. It opens up the possibility of APRV being a front-line ventilator modality for ARDS. However, we still need a robust, reproducible, multi-center RCTs to further confirm this conclusion.