Include excipient lung disease (ELD) in the differential diagnosis when evaluating and treating a patient with a history of intravenous drug use presenting with respiratory failure and typical findings on chest imaging.
CASE
A 38-year-old male presented to the emergency department via ambulance with a 4-day history of worsening agitation, confusion, altered mental status, fever and cough. The patient's wife reported seeing a text on the patient’s phone about possible recent intravenous injection of buprenorphine with naloxone. Upon arrival to the ED, the patient was afebrile (37.5°C) but extremely agitated. Initial vitals were notable for marked hypoxia (SpO2 74%) and tachycardia (140 bpm) with stable blood pressure (146/74 mmHg). On physical exam, the patient appeared to be a young, uncooperative man with diaphoresis, increased work of breathing, and accessory muscle use. Track marks were noted on his bilateral upper extremities. Due to worsening respiratory failure and agitation, the patient was emergently intubated. A CT angiogram showed a distinct tree-in-bud pattern with findings of pulmonary hypertension (Figure 1). The patient received vancomycin, piperacillin/tazobactam, and was admitted to the intensive care unit.
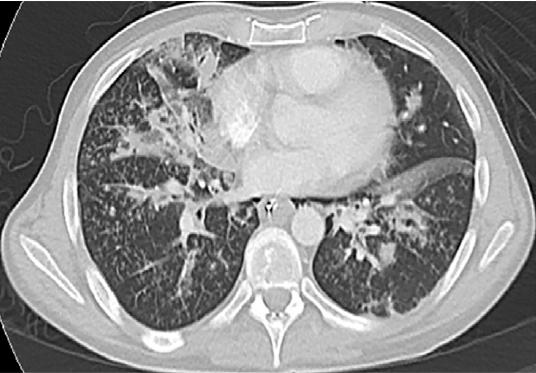
Figure 1. Axial cuts of computed tomography (CT) scan of patient’s chest suggesting excipient lung disease. Dashed circles indicate centrilobular periarteriolar micronodules that create a tree-in-bud pattern. Solid arrows show centrilobular micronodules in the lungs corresponding to perivascular granulomas, also often seen in excipient lung disease.
DISCUSSION
Excipient lung disease (ELD) occurs when foreign substance particles are lodged into pulmonary arterioles and capillaries and trigger a potentially fatal reaction referred to as pulmonary foreign body granulomatosis.1,2 The exact pathophysiology of this lung disease is based on the type of pulverized agent injected. Oral tablets contain excipients, which are insoluble particulate filler materials that bind and protect the active drug during production as well as shape/lubricate the tablet for easy swallowing. Common excipients include talc, microcrystalline cellulose, crospovidone, and starch.2 When these oral tablets are injected intravenously, these excipients may cause varying presentations of ELD. Patients with ELD are at increased risk of acute and chronic pulmonary complications such as pneumonia (10-fold increased risk), septic embolization, noncardiogenic pulmonary edema, foreign body granulomatosis, emphysema, interstitial lung disease, pulmonary vascular disease, pneumothorax, and increased incidence of fatal asthma exacerbation.3
Patients with ELD may initially present with nonspecific complaints such as dyspnea, cough, hypoxia with altered mental status. Advanced cases may present as acute respiratory disease syndrome (ARDS), panlobular emphysema, cor pulmonale, or acute pulmonary hypertension due to pulmonary arterial occlusion.4 In mild cases, the physical exam may reveal only bibasilar end-inspiratory crackles. Patients presenting with more advanced disease may show evidence of acute pulmonary hypertension, including augmentation of the second heart sound, a right ventricular heave, and/or peripheral edema.4 Physicians should also look for secondary signs of injection drug use such as needle marks, cutaneous abscesses, and hyperpigmented scars at the sites of previous injections.5
The initial evaluation of a patient with a history concerning for ELD should include chest radiograph, continuous pulse oximetry and arterial blood gas analysis. The chest radiograph in ELD typically shows widespread, small (2-3 mm) well-defined micronodules, often occurring in the midlung zones.6 The typical CT findings include numerous centrilobular micronodules in the lungs (Figure 1: solid arrows), corresponding to perivascular granulomas. Although centrilobular nodules typically reflect bronchiolar disease, in the setting of excipient lung disease, they reflect embolic arteriolar disease.7 Centrilobular periarteriolar micronodules can also appear as a tree-in-bud pattern (Figure 1: dashed circles), further mimicking bronchiolar disease.7 The “tree-in-bud pattern” described in the case refers to small centrilobular nodules of soft tissue attenuation connected to multiple branching linear structures of similar caliber that originate from a single stalk. (See Table 1 for differential diagnosis of "tree-in-bud" pattern on CT imaging.)
In addition to centrilobular nodules, other CT findings such as enlargement of the pulmonary arteries from pulmonary hypertension and other secondary signs of right heart strain should raise concern for excipient lung disease once pulmonary embolism and chronic pulmonary hypertension have been excluded.8,9 Echocardiography should be considered for further estimation of the pulmonary artery pressures and to rule out concomitant endocarditis.8 If a diagnosis is still unclear following CT imaging, a transbronchial biopsy may be performed, with the specimens being sent for microbiologic culture and histopathologic examination.10
MANAGEMENT OF ELD
The treatment of ELD is largely individualized and based on the severity of symptoms and degree of respiratory impairment. In patients who do not present with acute respiratory distress, supportive care measures may be sufficient to improve symptoms that can last from days to weeks. Following resolution of the acute episode, periodic reassessment with repeat chest imaging and echocardiography is recommended as secondary lung fibrosis and pulmonary hypertension may develop over months to years.8
The use of systemic steroids in patients who develop granulomatosis has limited supporting data and is generally not recommended.11 Patients with intravenous drug use should also be counseled on their habits, treated for associated illnesses, and referred for outpatient counseling when appropriate.
The foreign body granulomatosis associated with ELD can lead to increased long-term morbidity because of the complications of angiothrombosis, pulmonary hypertension, severe emphysema, chronic hypoxia, and progressive interstitial lung disease.8 It is also suggested that individuals who have injected a higher number of crushed pills may have a worse prognosis.4 Lung transplantation has been performed in patients with advanced pulmonary hypertension secondary to foreign body granulomatosis.12
CASE CONCLUSION
Given the initial CT findings, the patient underwent an extensive work-up for possible infectious causes. A bronchoscopy with bronchoalveolar lavage was notable for Methicillin-sensitive Staphylococcus aureus (MSSA) and blood cultures were positive for Streptococcus pneumonia and MSSA bacteremia. Respiratory PCR analysis was positive for Influenza A. A transthoracic echocardiogram was negative for valvular vegetations concerning for endocarditis but did reveal biventricular heart failure.
The patient was treated with oseltamivir for severe influenza and a prolonged course of antibiotics for sepsis secondary to bacterial pneumonia. The patient was eventually extubated on hospital day 10 without further complications. Repeat transthoracic echocardiogram showed normalization of biventricular function, and subsequent thoracic CT showed interval improvement of the tree-in-bud pattern. The patient was discharged to his home on hospital day 25 with outpatient referral for polysubstance abuse rehabilitation.
Excipient lung disease can manifest from many different injected substances and can present with clinical manifestations ranging from asymptomatic to acute respiratory failure. Knowing and recognizing radiologic patterns, in conjunction with the patient’s history and physical exam, will help emergency physicians and radiologists narrow the differential diagnosis and provide appropriate treatment.
Table 1. Differential Diagnosis of Tree-In-Bud Pattern on CT imaging3
|
Differential Diagnosis for "Tree-in-bud pattern" on CT imaging |
|
Bronchioalveolar Infection |
|
Congenital disorders (cystic fibrosis, Kartagener’s syndrome) |
|
Idiopathic disorders (obliterative bronchiolitis, panbronchiolitis) |
|
Aspiration pneumonitis |
|
Inhalation of foreign substances |
|
Immunologic disorders (allergic bronchopulmonary aspergillosis) |
|
Connective tissue disorders |
|
Neoplasms |
TAKE-HOME POINTS
- Include excipient lung disease (ELD) in the differential diagnosis when evaluating and treating a patient with a history of intravenous drug use presenting with respiratory failure and typical findings on chest imaging.
- Patients with acute presentations of ELD may also have superimposed bronchoalveolar infections, endocarditis, and acute pulmonary hypertension; ED clinicians should keep these in mind when evaluating these patients.
- Treatment for acute presentations ELD include airway/respiratory support as indicated and supportive care. Data on the use of steroids or other interventions is limited.
- Physicians should consider a wide differential for patients with tree-in-bud pattern on CT. (Table 1).
REFERENCES
- Wolff AJ, O’Donnell AE. Pulmonary effects of illicit drug use. Clin Chest Med. 2004;25:203-216.
- Tomashefski JF, Felo JA. The pulmonary pathology of illicit drug and substance abuse. Curr Diagn Pathol. 2004;10:413-426.
- Santiago ER, Franquet T, Volpacchio M, Gimenez A, Aguilar G. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. 2005;25:789-801.
- Pare JP, Cote G, Fraser RS. Long-term follow-up of drug abusers with talcosis. Am Rev Respir Dis. 1989; 139:233.
- Del Giudice P. Cutaneous complications of intravenous drug abuse. Br J Dermatol. 2004; 150:1.
- Genereux GP, Emson HE. Talc granulomatosis and angiothrombotic pulmonary hypertension in drug addicts. J Can Assoc Radiol. 1974; 25:87.
- Eisenhuber E. The tree-in-bud. Radiology 2002;222:771-772.
- Hind Cr. Pulmonary complications of intravenous drug use.2.Infective and HIV related complications. Thorax. 1990;45:957.
- Pintado V, Valencia ME, Lavilla P et al. [Angiothrombotic pulmonary granulomatosis in intravenous drug addicts]. Rev Clin Esp. 1991;188:362.
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008; 63 suppl 5:v1.
- Pare JA, Fraser RG, Hogg JC, et al. Pulmonary “mainline” granulomatosis: talcosis of intravenous methadone abuse. Medicine (Baltimore). 1979;58:229.
- Cook RC, Fradet G, English JC, et al. Recurrence of intravenous talc granulomatosis following single lung transplantation. Can Respir J. 1998;5:511.