Recently, a series of case reports have described the effect of COVID-19 on myocardial and pericardial tissue.
Case
A 56-year-old female with a history of CKD, multiple sclerosis, atrial fibrillation, DVT and PE on rivaroxaban, and recently recovered COVID-19 pneumonia presented to the ED due to sudden onset of mid-sternal chest pain that began earlier in the morning. The patient reported waking up in the middle of the night with left shoulder pain but then developed sudden heaviness in her chest. She was recovering from her COVID-19 pneumonia at a subacute rehab facility and had been doing well prior to these symptoms. Per medical records from her rehab facility, she did not miss any doses of her anticoagulation. A ROS was otherwise negative.
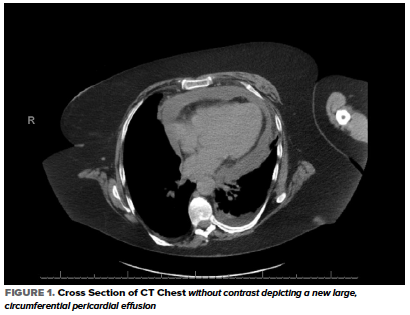
In the ED, her heart rate was 103 bpm, BP 123/67 mmHg, RR 24 breaths/min, temperature 36.7 C, and oxygen saturation 90% on 4L NC with no baseline oxygen requirement. Her physical examination was significant for conversational dyspnea, diminished breath sounds at the bilateral lung bases, and chronic bilateral lower extremity non-pitting edema. Blood work was significant for potassium of 5.6, creatinine 2.01, WBC 17.3, D-Dimer 948, CRP 240, LDH 324, and troponin <0.01. An EKG showed sinus tachycardia and chronic ST-segment changes unchanged compared with prior. A CT Chest without contrast showed interval development of a moderate to large simple appearing pericardial effusion with new small bilateral pleural effusions and associated compressive atelectasis (Figure 1).
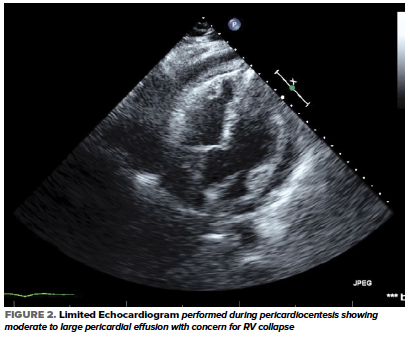
A subsequent bedside echocardiogram showed a circumferential pericardial effusion with concern for RV collapse (Figure 2) which warranted an emergent pericardiocentesis.
During the pericardiocentesis, 230 mL of serosanguinous fluid was removed. A pericardial drain was left in place and removed 2 days later after no further output was noted. Fluid studies were consistent with an exudative process with fluid LDH of 955 (ratio of fluid/serum LDH > 0.6). Bacterial, viral, and fungal cultures were all negative.
Given the patient's clinical history of recent COVID-19 pneumonia and lack of evidence of another infectious process, it was determined that the patient’s pericardial effusion was due to ongoing inflammation from COVID-19. The patient was treated with colchicine (NSAIDs were avoided given her initial presentation of an AKI with history of CKD) and there was no effusion seen on a repeat echocardiogram prior to discharge. She was continued on colchicine for a total of three months and remained asymptomatic on outpatient follow-up visits with cardiology.
Discussion
Acute pericarditis involves inflammation of the pericardial sac, which is made of an inner mesothelial visceral layer that surrounds the heart. Normally, this layer produces ~50 mL of fluid to lubricate the heart and prevent excess motion. The inflammation associated with pericarditis can lead to excess fluid production and the development of an effusion which can ultimately cause hemodynamic instability. Whether an effusion leads to cardiac tamponade is determined by both the volume of fluid and the rate of accumulation. Autoimmune diseases, neoplasms, uremia, and certain infections such as TB can cause large effusions that do not result in tamponade because their slow growth allows the pericardium to stretch and accommodate to the volume of fluid. Conversely, tamponade can develop with small effusions if the fluid accumulates over a short period of time.
Acute pericarditis typically presents with ≥ 2 of the following: sharp and pleuritic chest pain that is worse with laying down and improved with leaning forward, pericardial rub, new widespread STE or PR depressions, and new or worsening pericardial effusion. When acute pericarditis leads to a clinically significant effusion and cardiac tamponade, Beck’s Triad may be seen. This triad includes hypotension, jugular venous distention, and muffled heart sounds. Pulsus paradoxus, which is a decrease in systolic blood pressure by more than 10 mmHg with inspiration, can also be present in these patients. This happens when increased pressure from the effusion causes impaired ventricular relaxation and filling which leads to decreased preload and stroke volume.
Most cases of acute pericarditis in the world are idiopathic. However, for cases in which the cause is known, a virus is often to blame. In the pediatric population, coxsackie and echovirus are the most common viruses implicated, while in the adult population, CMV, HSV, and HIV infections are most implicated. Recently, a series of case reports have described the effect of COVID-19 on myocardial and pericardial tissue. It appears the majority of cases of myocarditis, pericarditis, and pericardial effusions secondary to COVID-19 are due to the overwhelming inflammatory reaction and infiltration rather than direct viral invasion.1
A case series published in the European Heart Journal presented three cases of pericarditis thought to be related to COVID-19 infection. Several viral, bacterial, and fungal tests were performed in each of the cases without any causative factors identified other than the patient being COVID-19 positive on RT-PCR testing. Interestingly, one of the cases tested the pericardial fluid for COVID-19 and was positive.2 In another case report of a patient with pericardial effusion and subsequent tamponade, it was determined that the systemic inflammatory response from the infection was the cause of the effusion. They noted that unfortunately there is no validated test to assess for COVID-19 in pericardial fluid, however the PCR testing they performed on the fluid was negative.3 In all cases published thus far, trials of NSAIDs and colchicine along with emergent pericardiocentesis led to resolution of the effusion and improvement of the symptoms related to pericarditis.
As prevalence of COVID-19 remains high, it is important to be aware of the possible cardiac effects of the infection in both the acute to subacute phases of the illness. Although further investigation is needed, it does appear that COVID-19 can lead to fulminant myopericarditis and subsequent cardiac tamponade and therefore requires us to maintain a high index of suspicion for this life-threatening diagnosis.
References
- Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127-31.
- Sauer F, Dagrenat C, Couppie P, Jochum G, Leddet P. Pericardial effusion in patients with COVID-19: case series. European Heart Journal - Case Reports. 2020;4(F11):1-7.
- Fox K, Prokup JA, Butson K, Jordan K. Acute Effusive Pericarditis: A Late Complication of COVID-19. Cureus. 2020;12(7):e9074.