Point-of-care ultrasound potentially saved a life in this case of cardiac tamponade in a patient with end-stage renal disease.
End-stage renal disease is increasing in the United States. These patients demonstrate an increased risk for all-cause mortality, most commonly from cardiovascular etiologies.1 Dyspnea is one of the most commonly reported symptoms by patients with chronic kidney disease (CKD). Observational studies have demonstrated that its prevalence in conservatively managed patients with ESRD is as high as 60%.2,3 Pulmonary edema and volume overload are the most common causes of dyspnea in this population.1 However, patients are also at risk for other causes of dyspnea, including acute coronary syndrome (ACS), unrecognized chronic lung disease, pulmonary hypertension, air embolism, hyperkalemia, anemia, pericarditis, and pericardial effusion.1,2
The incidence of pericardial disease in ESRD patients is < 20%, ranging from pericarditis to pericardial effusion to cardiac tamponade.4 Pericardial effusion is usually associated with fluid overload in this population.1 We present a case of cardiac tamponade in an ESRD patient, diagnosed with POCUS that prevented a potentially fatal outcome if the patient underwent hemodialysis.
Case
A 47-year-old male with a past medical history including CKD (recently started hemodialysis), coronary artery disease, and chronic obstructive pulmonary disease (normally on 2 L of oxygen) presented to the ED from hemodialysis with a 1-day history of moderately worsening shortness of breath. While at hemodialysis his oxygen saturations were 86-87% on 4 L of oxygen. The patient reported a cough and orthopnea over the past day, but he denied fever, chest pain, hemoptysis, and abdominal pain. He was hospitalized numerous times over the past few months and displayed poor compliance.
Upon arrival to the ED, his initial vital signs were temperature of 98.0 degrees Fahrenheit, heart rate of 121 bpm, respiratory rate of 24, blood pressure of 140/84, oxygen saturation of 89% on 4 L nasal cannula. The physical exam was notable for an ill-appearing male with labored respirations, conversational dyspnea, coarse bilateral inspiratory and expiratory wheezing, and diminished bibasilar breath sounds. He was tachycardic, had bilateral lower extremity swelling, and jugular venous distention. The patient was given bronchodilators, steroids, and his oxygen was increased to 5 L nasal cannula with improvement of oxygen saturation to 92%.
The initial plan consisted of an ECG, lab evaluation, portable chest radiograph, and computed tomography angiography (CTA) of chest. The ECG demonstrated sinus tachycardia. Laboratory evaluation demonstrated a white blood cell count of 18.9 K/mL, sodium of 140 mmol/L, potassium of 5.1 mmol/L, and creatinine of 5.5 mg/dL. His troponin was 0.03 ng/mL. A chest radiograph (Image 1) demonstrated diffuse bilateral interstitial and airspace disease most likely representing pulmonary edema with the possibility of a right-sided pneumonia. Cardiomegaly and pulmonary venous congestion were also demonstrated. Prior to ordering the CTA of chest, POCUS was performed.
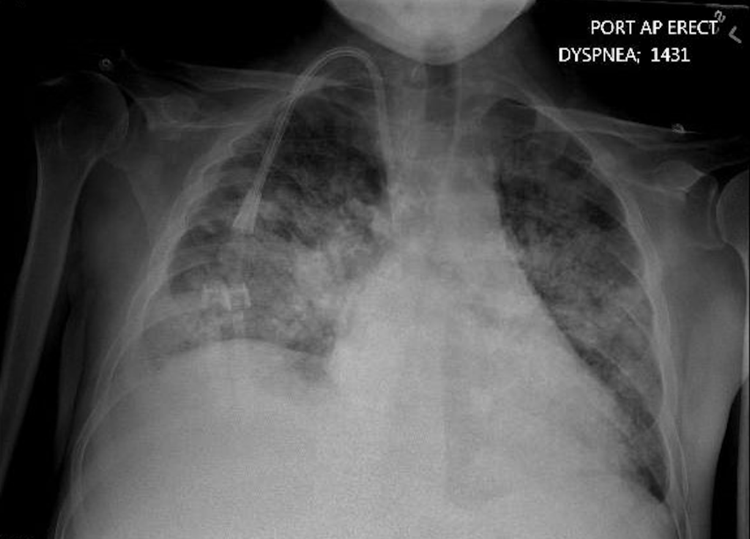
Image 1. Anterior-posterior chest radiograph demonstrating diffuse bilateral interstitial and airspace disease with cardiomegaly
POCUS demonstrated bilateral pulmonary edema and a moderate pericardial effusion with early tamponade physiology. The CTA of chest order was discontinued. Cardiology and nephrology were both consulted and felt the patient was too unstable to tolerate dialysis. The patient was admitted to the ICU, and thoracic surgery performed a pericardial window, removing 750 mL of serous fluid; findings were consistent with fibrinous uremic pericarditis. After stabilization, patient underwent dialysis and improved significantly.
Discussion
Cardiac tamponade is often thought of as a clinical diagnosis based on Beck's Triad: hypotension, dilated neck veins (JVD), muffled heart sounds.5 However, these findings are only seen in a minority of patients, and none of the findings alone are highly specific or sensitive.6 Although our patient had a moderate pericardial effusion and sonographic evidence of cardiac tamponade, he was normotensive. Studies have demonstrated that most patients with non-traumatic cardiac tamponade are normotensive (50%) or hypertensive (35%), with only 15% of patients being hypotensive.7 The physical exam is subjective and can vary among providers.8 Key physical exam findings, such as JVD and muffled heart sounds, may be variable among providers.
The workup of ECG, laboratory evaluation, and chest radiograph did not provide much usefulness in establishing this patient's diagnosis. In fact, the workup may have been misleading and committed the patient to an intervention that would have been harmful (hemodialysis).
Sinus tachycardia is very common in cardiac tamponade, but is not nearly as specific as ECG findings of electrical alternans or low QRS voltage.9,10 Chest radiographs are neither specific nor sensitive for cardiac tamponade. Findings of cardiomegaly, as in this patient, are non-specific for cardiac tamponade and can be attributed to other disease processes, such as congestive heart failure.6,9 Furthermore, the chest radiograph in the case demonstrated findings consistent with pulmonary edema - which could have easily, but incorrectly, led the provider to attribute the patient's dyspnea to volume overload. Lastly, POCUS also prevented the patient from undergoing a CTA of chest. Although a CTA of chest can provide useful information for the diagnosis of cardiac tamponade, it would have exposed the patient to unnecessary radiation and delayed the appropriate therapy.11
POCUS has been proven useful in the ED for managing critically ill patients and is highly recommended for patients with suspected pericardial disease.6,12 POCUS diagnosis of cardiac tamponade is suggested by:13,14
- Diastolic chamber collapse
- Increased respiratory variations of transmitral and transtricuspid doppler inflow velocities
- Inferior vena cava (IVC) plethora
Diastolic collapse of the right heart is highly suggestive of cardiac tamponade.7 Diastolic collapse of the right atrium is more specific and sensitive compared to diastolic collapse of right ventricle, especially if the right atrium is collapsed for greater than a third of the cardiac cycle.15,16 Respiratory variation of flow velocities across mitral and tricuspid valves are indicative of cardiac tamponade. When referenced to expiration, patients with cardiac tamponade will typically have a flow of variation of > 30% and 60% across the mitral valve and tricuspid valve respectively.17 Lastly, IVC plethora (defined as IVC dilatation plus < 50% IVC diameter reduction during inspiration) is highly sensitive, but not specific for cardiac tamponade.18 The patient in our case had, right atrial collapse for > 50% of cardiac cycle, transmitral flow velocity variation of 30.4%, and IVC plethora.
This case demonstrates an ESRD patient with dyspnea secondary to a nonclassic presentation of cardiac tamponade. Despite chest radiograph evidence suggesting volume overload and pulmonary edema, ESRD patients are a unique population that can have dyspnea for nonvolume related complications. Without POCUS, this patient could have received suboptimal management by undergoing hemodialysis instead of receiving the more urgently needed pericardial window. Based on the broad pathology associated with ESRD, the authors will have an increased index of suspicion for pericardial disease in dyspneic patients.
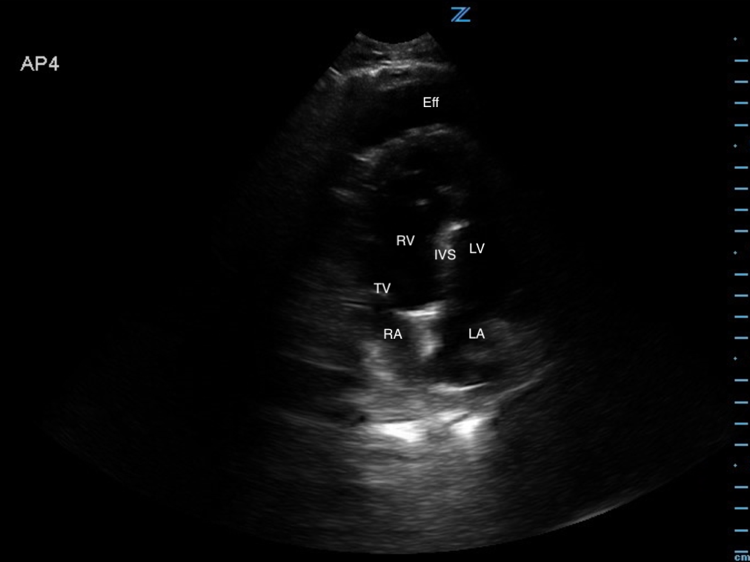
Image 2. Abnormal POCUS (apical chamber) view in 2D mode demonstrates a large pericardial effusion with right atrial collapse during diastole. [Eff = effusion; RV = right ventricle; IVS = intraventricular septum; LV = left ventricle; TV = tricuspid valve; RA = right atrium; LA = left atrium]
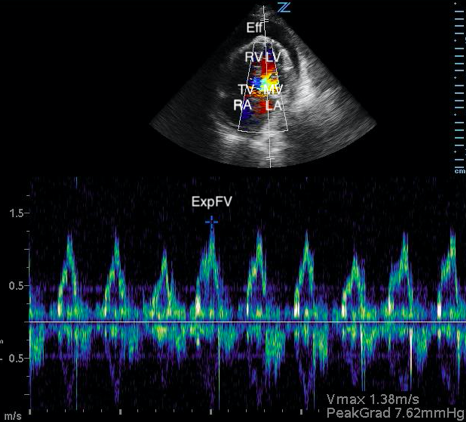
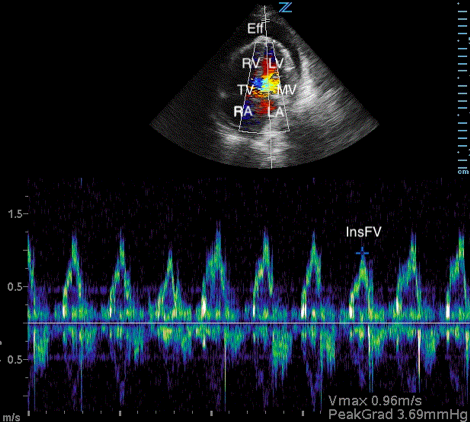
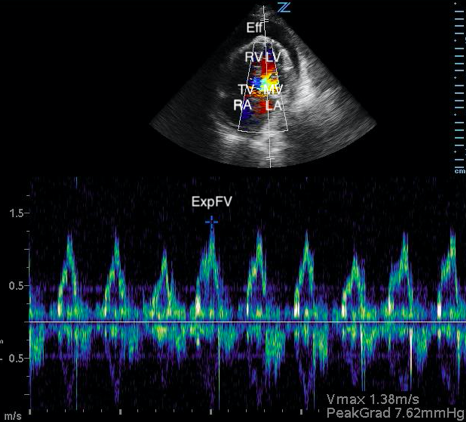
Image 3. Abnormal POCUS (apical 4 chamber) view in 2D mode with color flow doppler demonstrating abnormal flow velocity across mitral valve during respiration (30.4%). [Eff = effusion; RV = right ventricle; LV = left ventricle; TV = tricuspid valve; MV = mitral valve; RA = right atrium; LA = left atrium; ExpFV = expiratory flow velocity; InsFV = inspiratory flow velocity]
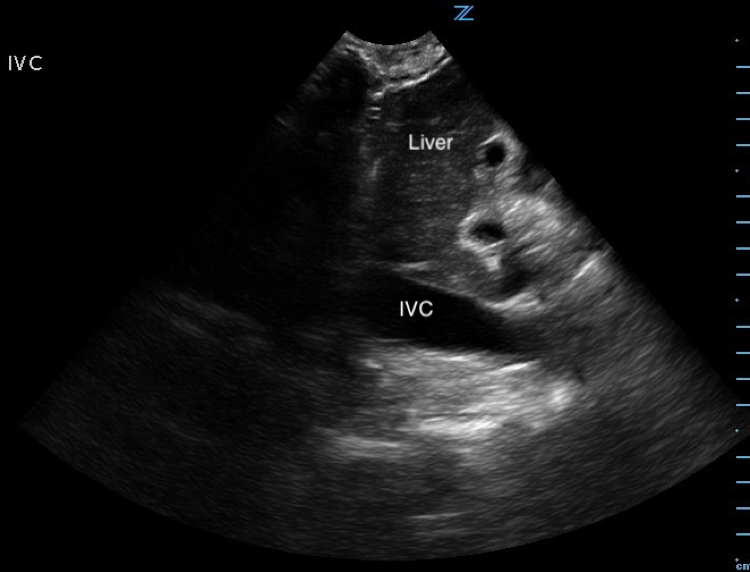
Image 4. Abnormal POCUS (longitudinal subcostal view in 2D-mode) demonstrating inferior vena cava plethora. [IVC = inferior vena cava]
References
1. Long B, Koyfman A, Lee CM. Emergency medicine evaluation and management of the end stage renal disease patient. Am J Emerg Med. 2017 Dec;35(12):1946-1955.
2. Salerno FR, Parraga G, McIntyre CW. Why is your patient still short of breath? understanding the complex pathophysiology of dyspnea in chronic kidney disease. Semin Dial. 2017 Jan;30(1):50-57.
3. Murtagh FEM, Addington‐Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross‐sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007; 10(6):1266–1276.
4. Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci. 2003 Apr;325(4):228-36.
5. Beck CS. Two cardiac compression triads. J Am Med Assoc. 1935;104:714.
6. Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–2964.
7. Saul T, Siadecki SD, Berkowitz R, et al. M-mode ultrasound applications for the emergency medicine physician. J Emerg Med. 2015;49(5):686-692.
8. Kamin RA, Nowicki TA, Courtney DS, et al. Pearls and pitfalls in the emergency department evaluation of abdominal pain. Emerg Med Clin North Am. 2003;21(1):61-72.
9. Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349(7):684.
10. Bruch C, Schmermund A, Dagres N, Bartel T, Caspari G, Sack S, Erbel R. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38(1):219.
11. Azarbal A, LeWinter MM. Pericardial effusion. Cardiol Clin. 2017 Nov;35(4):515-524.
12. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). Circulation. 2003 Sep 2;108(9):1146-1162.
13. Plotnick GD, Rubin DC, Feliciano Z, Ziskind AA. Pulmonary hypertension decreases the predictive accuracy of echocardiographic clues for cardiac tamponade. Chest. 1995;107(4): 919.
14. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673.
15. Gillam LD, Guyer DE, Gibson TC, King ME, Marshall JE, Weyman AE. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation. 1983;68(2):294.
16. Kerber RE, Gascho JA, Litchfield R, Wolfson P, Ott D, Pandian NG. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982;307(15):929.
17. Klein AL, Abbara S, Agler DA, et al. American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. J Am Soc Echocardiogr. 2013;26(9):965.
18. Mercé J, Sagrista-Sauleda J, Permanyer-Miralda G, Evangelista A, Soler-Soler J. Correlation between clinical and doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138(4 Pt 1):759.