As emergency medicine providers, how can we best approach the cyanotic and non-cyanotic infant?
Cases
- A 5-day-old boy presents with central cyanosis, mild tachypnea, and lethargy. SpO2 was 50-60% and did not improve after 10 minutes of oxygen. Physical exam revealed a grade III/VI systolic murmur at the lower sternal border without rales or wheezing. The liver was palpable and electrocardiogram (EKG) showed right axis deviation, a tall p-wave and right ventricular hypertrophy. Chest x-ray (CXR) showed cardiomegaly with black lung fields.1
- A 4-month-old girl, ex-37 weeker presents with 2 days of increased work of breathing and poor feeding. She has no fever, cough, or diarrhea, but has siblings with colds. At her 2-month check-up, a diastolic murmur was noted but has not been followed up. Mom states she recently spends >40 minutes feeding per breast and appears sweaty afterward. Vitals include a respiratory rate of 60/min and a SpO2 of 90% on room air. On physical exam, you note respiratory distress with scattered rales, rhonchi, and wheezing. You consider bronchiolitis, but decide to obtain a CXR which shows cardiomegaly and patchy perihilar opacities.2
- A 7-day-old boy, born full-term at home by a midwife with minimal prenatal care, presents by ambulance with fatigue during feeding and “fast breathing” for 1 day. He appears ashen and limp, with vital signs of 36.2oC, pulse of 198 beats/min, breathing 80 breaths/min on room air at 92% SpO2. Your differential includes sepsis, metabolic disease, and congenital heart disease. You initially order broad-spectrum antibiotics, fluids, chest radiography, and a bedside transthoracic echocardiogram (TTE) but consider whether empiric prostaglandins (PGE1) are safe to give prior to receiving the results of the workup.2
As emergency medicine providers, how can we best approach the cyanotic and non-cyanotic infant? The most common categories of lesions that present to the ED are:
- Right-sided obstructive ductal-dependent
- Left-sided obstructive ductal dependent
- Shunting or mixing lesions
However, these are most easily thought of in terms of age and degree of cyanosis. Strobel and Lu created a simple system for the presenting pink, blue, and gray baby (Table 1).3
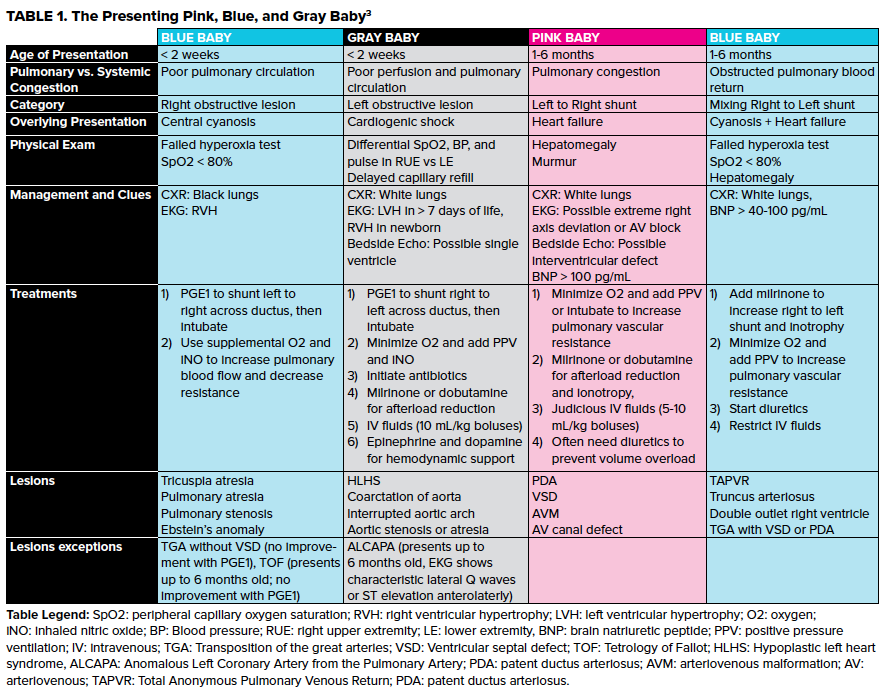
Presentation to the ED
The approach to an infant with suspected congenital heart disease is multi-faceted. These patients can present with shock, cyanosis, tachypnea, and pulmonary edema. To differentiate central vs peripheral cyanosis in an infant, especially in darker skin tones, examine the gum-lines and tongue for purple-blue hues. Isolated circumoral cyanosis may be an indicator of peripheral cyanosis.4 A cyanotic infant differential diagnosis must include:
- CHD
- Sepsis
- Respiratory disorders
- Hematologic disorders such polycythemia and methemoglobinemia
Respiratory disorders are often difficult to distinguish from cardiac causes in the neonate, especially when a CHD presentation may exclude an audible murmur and may not be reflective of the older child presentation, such as dyspnea on exertion, exercise intolerance, syncope, or abdominal pain. CHD infants instead will exhibit irritability, sweating, crying with feeds, failure to thrive, and “quiet tachypnea.” Alternatively, an infant with a respiratory disorder may have similar features but will generally respond to oxygen and have positive lung field findings on chest radiograph. The most sensitive and specific variables (p < 0.0001) for congestive heart failure (CHF) include a history of feeding < 3 oz or spending > 40 minutes per breast, an abnormal respiratory pattern with resting rate >60/min, a diastolic murmur, and hepatomegaly.5
The hyperoxia test is another highly effective method of distinguishing respiratory from cardiac tachypnea.6 The standard approach tests PaO2 from serial ABGs before and after 10 minutes of 100% O2, with a rise above 150 mmHg indicating a respiratory cause. A modified approach measures pulse oximetry before and after 10 minutes of 100% O2. With tachypnea in suspected CHD, assess differentials of pulse oximetry, pulse, and blood pressure in the pre-ductal right arm, and either leg. A ductal dependent lesion is suggested with a differential in >3% on pulse oximetry, in discrepancy of pulse strength palpation, or in mean arterial pressure (MAP) > 20 mmHg.4
Pearl: What is the best objective measure of distinguishing between respiratory and cardiac cause of tachypnea?
The modified hyperoxia test measures pulse oximetry before and after 10 minutes of 100% O2, and is a rapid alternative compared to serial ABGs of the standard hyperoxia test.
Management and Treatment
Initial diagnostic work-up for suspected congenital heart disease in infants should include EKG, CXR and bedside TTE. A pediatric EKG may show sinus tachycardia, left ventricular hypertrophy, ST-T changes, first degree AV block or inferolateral Q-waves, suggesting ALCAPA.6 Classic CXR findings include Snowman sign of TAPVR, the Boot-shaped heart in TOF, and the egg-on-a-string sign in TGA. Of note, a right aortic notch with leftward tracheal deviation is associated with CHD 90% of the time.7 A black lung field in a hypoxic neonate with metabolic acidosis suggests a right-sided obstructive lesion, whereas white lung fields suggest pleural effusions in left to right shunts. Finally, bedside TTE will provide a quick determination structural or functional lesions are present.
Pearl: Can any labs help distinguish worsening heart failure in the setting of respiratory illness?
Consider brain natriuretic peptide (BNP or amino-terminal [NT]-proBNP) for distinguishing between cardiac and respiratory etiology.8 In a recent study, a BNP of 95 pg/ml (sensitivity: 0.71, specificity: 0.91, positive predictive value: 0.83) was the optimal cutoff for concern of heart failure. However, note that plasma BNP levels are higher in patients with left ventricular volume overload compared to patients with right ventricular pressure or volume overload.9
Following initial stabilization and management, the ED treatment is clinical presentation guided. In the blue neonate, focus on lowering pulmonary vascular resistance (PVR) for improved pulmonary blood flow (PBF) by providing supplemental oxygen and inhaled nitric oxide (iNO). When a right-sided obstructive lesion with a closing ductus arteriosus is suspected in a cyanotic neonate, start PGE1 and intubate. PGE1 can be started with an initial dose of 0.05 mcg/kg/min and titrated up to a maximum of 0.1 mcg/kg/min q15-20 min.10 If the patient begins to deteriorate after the initial dose, discontinue the infusion as this could signify pulmonary venous or left atrial obstruction as seen in TAPVR, or an infant with TGA without VSD or TOF.
Pearl: What is the preferred induction agent?
Etomidate is the agent of choice for patients in decompensated shock. Delayed side effects include seizures and decreased adrenocortical function, however adrenal reactions have been regarded as not clinically significant following a single dose11. Fentanyl is the preferred second line agent. Avoid ketamine in induction if CHD is suspected as it can increase systemic vascular resistance, worsen left to right shunts and lead to cardiovascular collapse.
Pearl: When are prostaglandins indicated?
PGE1 should be started as soon as possible in neonates with suspected CHD in the first month of life. In infants > 1 mo, they can cause apnea and hypotension. For neonates whose CHD improves with PGE1 administration, an effect should be seen within about 10 minutes in the blue baby and may take several hours in the gray baby. NEVER walk away after giving prostaglandin. Have a ventilator present and be ready for intubation.
For the gray neonate in cardiogenic shock, a left obstructive lesion may also be presenting in shock due to closure of the ductus arteriosus. In the ED, we should attempt to reopen the PDA to improve shunting from right to left and reduce afterload, as well as minimize oxygen consumption, add iNO and PPV. To reduce afterload, we can use milrinone or dobutamine. Use caution in giving milrinone in the hypotensive patient, as it can cause peripheral dilation due to vasodilatory properties.
Treatment goals for the pink infant with left to right shunt and pulmonary congestion involve increasing PVR. Lesions can be caused by a PDA, VSD, and AV canal defect. To decrease PVR, focus on minimizing oxygen levels and add PPV. Afterload should be reduced with milrinone or dobutamine, and they may benefit from diuretics to lessen pulmonary congestion and slow judicious 5-10 mL/kg IV fluid boluses as needed.
Lesions of the blue infant with CHF due to a mixing right to left shunt should focus on increasing PVR by further increasing the right to left shunt. Where PVR is low, PBF will be excessive and should be restricted with ventilatory manipulations to achieve balanced circulation. Ventilator settings should aim for a SaO2 of 70-85% with a relatively normal pCO2 of 35-45 mmHg. PGE should not be given in this instance, as it will increase left to right shunt across the PDA, thereby increasing pulmonary blood flow. Shunt blood away from the lungs by mildly hypoventilating, add PPV and decrease supplemental oxygen. Milrinone will also improve right to left shunting and ionotropy.
CHD can predispose pediatric patients to numerous infections, including infective endocarditis,12 with the most common being gastroenteritis and bronchiolitis, in parallel with the general pediatric ED population.13 Arrhythmias are common as well, with the most common being tachyarrhythmias including atrial flutter, atrial fibrillation, and paroxysmal ventricular tachycardia.12,14
Case 1 Conclusion
This patient presented with central cyanosis at < 2 weeks, indicating a high probability of right obstructive lesion, worsening due to closure of the ductus arteriosus. The lesion is reliant upon the ductus to provide blood flow to the pulmonary artery and as it closes, the infant develops progressive respiratory distress and cyanosis which is not improved with oxygen administration. Management included intubation, increased oxygen to lower pulmonary vascular resistance, and PGE1. Bedside TTE in this case revealed severe tricuspid regurgitation and functional pulmonary atresia, most commonly seen in neonates with Ebstein’s anomaly. This patient will require balloon valvuloplasty or surgical repair.
Case 2 Conclusion
Despite initially suspecting bronchiolitis as the cause of the respiratory symptoms, you are suspicious of the cardiomegaly, history of murmur, and prolonged feeding with failure to thrive. You reexamine the patient and this time note hepatomegaly. You order a BNP which returns as elevated, further supporting a diagnosis of CHF. After ordering furosemide, the patient has a significant urine output leading to resolution of her tachypnea and improvement of SpO2 to 95% on room air. An echo confirms a diagnosis of large VSD with left to right shunt.
Case 3 Conclusion
You further your physical exam with an assessment of a blood pressure differential on the right arm and right leg. The patient has a significant reading of 84/40 in the arm and 60/32 in the leg. As a coarctation of the aorta seems most likely, you start a PGE1 infusion. Within 10 minutes, you notice improvement in capillary refill while a bedside echo confirms critical coarctation of the aorta. You admit the patient for surgical correction.
Pearls and Pitfalls
-
Be judicious about oxygen and fluids on CHD patients.
-
Prostaglandins should be given for any patient < 1 month old with suspected CHD, however never walk away once PGE1 is given without being prepared to intubate.
-
A modified hyperoxia test is preferred for distinguishing between a respiratory and cardiac cause of tachypnea.
-
Etomidate is first line for intubation induction.
-
BNP can be used for distinguishing between a cardiac and respiratory presentation, and a cutoff point of over 95 pg/mL is concerning for heart failure.
Acknowledgements
Special thanks to Nick Sausen, MD, and Anna McFarlin, MD, of LSUHSC Department of Emergency-Pediatrics for their wisdom and advice on the topic.
References
1. Lee JY. Clinical presentations of critical cardiac defects in the newborn: Decision making and initial management. Korean J Pediatr. 2010;53(6):669-679.
2. Judge P, Meckler G. Congenital heart disease in pediatric patients: Recognizing the undiagnosed and managing complications in the emergency department. Pediatr Emerg Med Pract. 2016:13(5):1-26.
3. Strobel AM, Lu le N. The Critically Ill Infant with Congenital Heart Disease. Emerg Med Clin North Am. 2015;33(3):501-518.
4. Joubert G, Strobel A. Congenital heart disease emergencies. Emergency Medicine Cases Podcast. Episode 84. August 2016.
5. Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72-75.
6. Clark C. The Pediatric ECG and Long QT Syndrome. EM Resident. 2018;45(5):18-19.
7. Schweigmann G, Gassner I, Maurer K. Imaging the neonatal heart–essentials for the radiologist. Eur J Radiol. 2006;60(2):159-170.
8. Kantor PF, Lougheed J, Danca A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian cardiovascular society guidelines. Can J Cardiol. 2013;29(12):1535-1552.