Background
Patients with end-stage systolic heart failure (defined as New York Heart Association Class IV or Ejection Fraction <25%) have high mortality and limited treatment options for this disease.1 Given the limited supply of donor hearts available for transplant, LVADs have emerged as a revolutionary treatment to bridge these patients to transplantation. More recently, LVADs were approved as a destination therapy for end-stage heart failure patients who were not candidates for heart transplantation.2
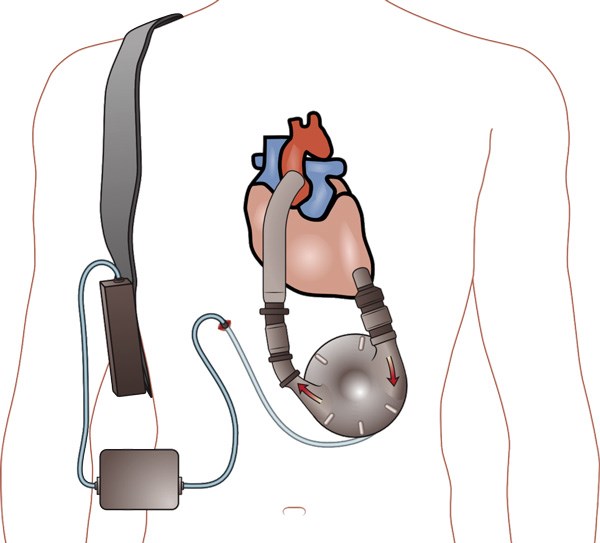
LVADs initially were pulsatile flow pumps that proved to be too bulky and demonstrated a 2-year failure rate above 70%.1 Second generation LVADs are continuous flow pumps with a rotary motor, and have been used almost exclusively since 2012.3,4 Thoratec HeartMate II and HeartWare HVAD are the most common LVADs currently used. LVADs are implanted in the thorax with the inflow cannula taking blood from the weakened left ventricle and pumping it into the ascending aorta. This pump receives its power supply via a driveline that runs subcutaneously and exits the body to a controller connected to a battery. The controller is the point where you can see the parameters from the LVAD, including pump speed, power, estimated blood flow, and pulsatility index.5
The general approach to these patients is twofold:
- Airway, breathing, circulation, IV, O2, monitor as you do with any patient.
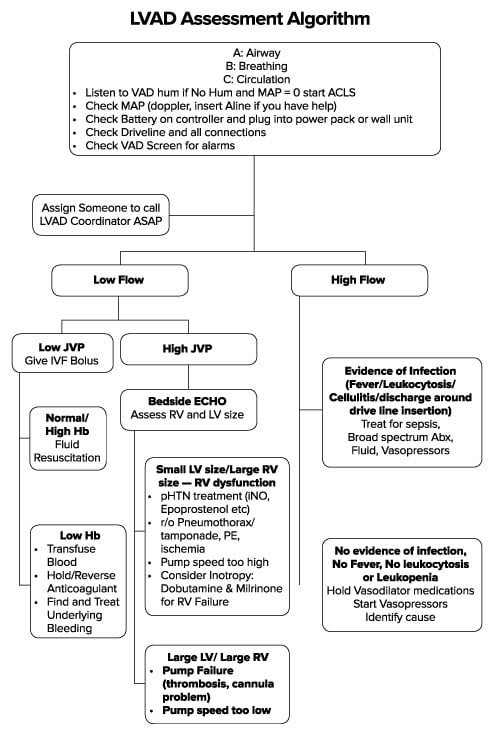
- Assessment of the LVAD (Figure 1).
When you examine the device, the first step is to listen for the VAD “hum” to see if it is running, followed by a check of the battery level. Most patients will come with backup batteries and/or a cord. The next step is to examine the controller box and see if there are any alarms active that can help guide therapy. With regard to vital signs, automatic blood pressure cuffs may be inaccurate because of the low pulse pressures in LVAD patients.4 A manual cuff and Doppler ultrasound may be necessary to estimate systolic blood pressure. Finally, you may need to call the LVAD team, often led by a nurse coordinator who manages outpatient LVAD concerns. This person can be helpful in notifying cardiology, arranging for an inpatient bed, and expediting overall care.
Following are some patient cases and associated LVAD complications.
Case 1. A 51-year-old male with history of nonischemic cardiomyopathy status post LVAD 6 months ago presents with episodes of lightheadedness. Of note, patient had his warfarin dose decreased about one month ago secondary to a GI bleed and symptomatic anemia requiring 3U pRBCs. What are the crucial steps in management?
Thrombosis and Bleeding
As with any patient on systemic anticoagulation, there is a balance between the risk of bleeding and the risk of thrombosis. LVAD patients are generally on antiplatelet agents and anticoagulation. The target INR for patients on warfarin is usually 2.0-3.0. As expected, patients with elevated INRs have an increased risk of bleeding events often manifesting as epistaxis, melena (often from arteriovenous malformations), or altered mental status (intracranial bleed)5. These are treated by directing therapy at the underlying cause. Occasionally, this may require reversing the coagulopathy with fresh frozen plasma or prothrombin complex concentrate, but this increases the risk for device thrombosis and embolism. Also, shear forces from the LVAD rotor cause destruction of the large von Willebrand factor monomers and in turn causes an acquired von Willebrand disease which further increases bleeding risk.3,5-7 These patients will benefit from cryoprecipitate or desmopressin. Platelet transfusions may also be required for decompensating thrombocytopenic patients. The LVAD control box will be alarming for low flow or low pulsatility index, which are consistent with undifferentiated hypovolemia (Table 1).5
Conversely, patients with sub-therapeutic INRs are at risk of thrombosis. Even patients with therapeutic INRs are at risk of thrombosis because the chronic low-level hemolysis leads to an increase in reactive oxygen species causing increased platelet activation, vascular tone, hypofibrinolysis and net hypercoagulability that cannot be offset by the natural heme-scavenging mechanisms.3 Diagnosis of this complication is critical and multiple laboratory markers have been studied with consensus placed on using LDH.3 Thrombosis should be considered if LDH is greater than 2.5 times the upper limit of normal at your institution.3,5 However, given that the LDH is expected to be chronically elevated because of low-level hemolysis, this diagnosis needs to be made in conjunction with signs of pump malfunction or alarms for increased pump power (Table 1).3,5 Treatment should be made in conjunction with the LVAD team and often results in temporary heparin infusions or thrombolysis in an unstable patient.
Table 1. Common LVAD Alarms (adapted).6,12*
Cardiology can adjust speed on LVADs to affect the parameters below. Speeds vary by device (HeartMate II LVAD 6,000-15,000 RPM, HeartWare HVAD 1,800-4,000 RPM7)
| Alarm | Meaning | Treatment |
| Battery | Low battery or battery malfunction | Switch to backup battery or plug device into outlet |
| High Flow | LVAD is trying to compensate for a vasodilatory state | Diagnose and treat presumed sepsis. Consider vasopressor |
| Low Flow/Suction Event | Hypovolemia/Bleeding/Arrhythmia | Bolus fluid/Transfuse Blood/ Treat Arrhythmia |
| High Power | Pump thrombus | Heparin – (possibly thrombolysis if life-threatening12) |
| Low Power /Low Pulsatility Index | Pump failure/disconnection
Hypovolemia/suction event Myocardial Ischemia |
Check connections, intravenous fluids to increase preload, inotropic support |
*Discuss all LVAD patients with their primary team
Case 2. A 62-year-old female with a history of non-ischemic cardiomyopathy status post LVAD 12 months ago presents with expressive aphasia upon waking from sleep. The patient noticed a similar episode last night, which resolved spontaneously within 2 hours. On physical exam, the patient is found to have focal neurologic deficits. The CT scan does not show any acute abnormalities. How should the clinician manage this patient?
Cerebrovascular Accidents
While systemic anticoagulation reduces the risk of thromboembolic events, there is a significant rate of cerebrovascular events reported in the literature that range from 10 to 25%, with nearly two-thirds involving the right hemisphere.8,9 Interestingly, the risk of ischemic stroke increased in patients with concomitant infection, thought to be related to the infection-induced hypercoagulable state.8 Treatment options are limited in this population and decisions on thrombolytics or neurointerventional radiology must be made between cardiology, neurology, and emergency providers.
Case 3. A 63-year-old female with a prior stroke and residual aphasia and visual deficits, non-ischemic cardiomyopathy status post LVAD, VAD thrombosis, and LVAD exchange presents with multiple syncopal episodes and 3 days of diarrhea, and LVAD is alarming for suction alarms. How should the clinician approach this patient?
Suction Events
A suction event refers to decrease in blood volume leading to increased negative pressure in the left ventricle causing wall collapse over the inflow cannula.1,5 These patients present with low mean arterial pressures and low flow alarms, signifying that either the heart is not supplying the LVAD enough blood or the LVAD is not pumping properly.1,5 Treatment is primarily fluid resuscitation and is etiology-focused. LVADs are preload dependent, and the left ventricle preload is dependent on the right ventricle (RV), so anything that causes worsening in RV flow or contractility can decrease LVAD function and forward flow. Worsening pulmonary hypertension (hypoxia, hypercarbia), pulmonary emboli, arrhythmias, RV ischemia, tension pneumothorax, or pericardial tamponade can cause severe RV failure.4 In some cases, patients will need inotropic support with milrinone, epinephrine, or dobutamine until the underlying cause is reversed.
Case 4. A 56-year-old patient with history of ischemic cardiomyopathy presents 8 months after implantation of LVAD with 3 days of pain, erythema, and discharge from the driveline site. What is your approach?
Infection
Infections can occur at any time but are most common in the first 3 months after device placement.5 The incidence of driveline infections has been reported to be as high as 30%.10 The most easily recognized clinical sign of a driveline infection is drainage from the entry site with surrounding cellulitis, but severe infections can involve the pump pocket or the pump itself. This can lead to pump endocarditis.5 CT scans can help to identify fluid collections, but will be limited by device artifact. Ultrasound may be helpful to detect fluid pockets, but this still comes with its limitations in proving whether the device is actually infected.5,10
Treatment should include broad spectrum antibiotics to target the most common pathogens such as Staphylococcus and gram negative organisms like Klebsiella or Pseudomonas.5,10 Sources vary on fungal coverage, but it has been reported in nearly 10% of patients.5,10 Surgical exploration is often necessary for definitive treatment.
Case 5. A 53-year-old man with a history of idiopathic cardiomyopathy is brought in by his wife 3 weeks after transplantation of a LVAD, after multiple episodes of low flow alarms at home. In triage, his EKG shows ventricular tachycardia. His device is alarming, but he is sitting up talking to you.
Dysrhythmia
These patients are at high risk for dysrhythmias given the underlying heart disease that led to LVAD placement. Luckily, these patients often tolerate dysrhythmias well because the device supports cardiac output. It is important to note, however, that dysrhythmias may impair right heart function, which will decrease cardiac output, and may lower the LV preload, and in return drop the LVAD flow. LVAD patients can be managed per regular ACLS algorithms. Pad placement for emergent cardioversion is recommended in an anterior and posterior placement to avoid the LVAD and driveline.6 Dysrhythmias can be triggered by postsurgical scarring, cannula migration, or suction events secondary to hypovolemia (diuresis, bleeding).5,7 Ventricular dysrhythmias are most common, and amiodarone is considered first line therapy, followed by lidocaine and procainamide. A fluid challenge may also be appropriate, followed by bedside echocardiogram to help guide further treatment in consultation with the LVAD team.
Case 6. Overhead page: EMS en route with a 42-year-old LVAD patient, vitals unstable, device is reading low flow, ETA 5 minutes. What is your plan?
Shock/Cardiac Arrest Case
While cardiac arrests are not unfamiliar to emergency physicians, LVADs pose additional considerations. To start, device manufacturers advise chest compressions only if necessary, given the risk of dislodging the cannulas. That said, some early evidence suggests this may be theoretical and compressions are not absolutely contraindicated for patients in extremis.5,11
A recently published Scientific Statement for the American Heart Association states that, “A PETCO2 (Partial Pressure End-Tidal CO2) value of <20 mm Hg in an unresponsive, correctly intubated, pulseless patient with a left ventricular assist device (LVAD) would seem to be a reasonable indicator of poor systemic perfusion and should prompt rescuers to initiate chest compressions.”4
Rapid device assessment is essential to triage a device cause or non-device cause. Ensure the device is running by listening for the LVAD “hum” over the left chest and left upper abdominal quadrant, checking the battery and blood flow/manual blood pressure.
Look at the cardiac rhythm. Dedicate a person to check the device connections: are the lines secure? Is the battery alive? Is the power source connected? Does the controller need to be replaced? Look at the alarm! Assess tissue perfusion using the usual clues of skin temperature, appearance, mental status and capillary refill. These patients may benefit from an arterial line to help guide resuscitation, in addition to ETCO2. Unconscious patients with undetectable pulse but good MAPs likely have a functioning LVAD.
A good history and physical in conjunction with a broad workup and discussions with the LVAD team are needed to provide care for these complex patients.
References
- Caraang C, Lanier GM, Gass A, et al. Left Ventricular Assist Device in Older Adults. Heart failure clinics. 2017;13(3):619-632.
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361(23):2241-2251.
- Shah P, Tantry US, Bliden KP, et al. Bleeding and thrombosis associated with ventricular assist device therapy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017.
- Peberdy MA, Gluck JA, Ornato JP, et al. Cardiopulmonary Resuscitation in Adults and Children With Mechanical Circulatory Support: A Scientific Statement From the American Heart Association. Circulation. 2017;135(24):e1115-e1134.
- Greenwood JC, Herr DL. Mechanical circulatory support. Emergency medicine clinics of North America. 2014;32(4):851-869.
- Partyka C, Taylor B. Review article: ventricular assist devices in the emergency department. Emergency medicine Australasia : EMA. 2014;26(2):104-112.
- Pratt AK, Shah NS, Boyce SW. Left ventricular assist device management in the ICU. Critical care medicine. 2014;42(1):158-168.
- Kato TS, Ota T, Schulze PC, et al. Asymmetric pattern of cerebrovascular lesions in patients after left ventricular assist device implantation. Stroke. 2012;43(3):872-874.
- Tsukui H, Abla A, Teuteberg JJ, et al. Cerebrovascular accidents in patients with a ventricular assist device. The Journal of thoracic and cardiovascular surgery. 2007;134(1):114-123.
- Hernandez GA, Breton JDN, Chaparro SV. Driveline Infection in Ventricular Assist Devices and Its Implication in the Present Era of Destination Therapy. Open journal of cardiovascular surgery. 2017;9:1179065217714216.
- Shinar Z, Bellezzo J, Stahovich M, et al. Chest compressions may be safe in arresting patients with left ventricular assist devices (LVADs). Resuscitation. 2014;85(5):702-704.
- Sen A, Larson JS, Kashani KB, et al. Mechanical circulatory assist devices: a primer for critical care and emergency physicians. Critical care (London, England). 2016;20(1):153.