Wernicke’s encephalopathy can have a subtle presentation and should be considered in any patient with altered mental status who is at risk for nutritional deficiency.
Introduction
Wernicke’s encephalopathy (WE) is named after Carl Wernicke. More than 100 years ago, the German physician noted a combination of ophthalmoplegia, ataxia, and “mental compromise” in a woman with chronic malabsorption from severe pyloric stenosis.1
Since then, a deficiency of thiamine (vitamin B1) has been determined to be the primary factor causing WE, with alcohol abuse being the primary etiology of thiamine deficiency considered.2 However, there are numerous other conditions that predispose patients to thiamine deficiency, and these are not always obvious to the clinician.
Case
A 45-year-old female with obesity and a past medical history of hypertension and obstructive sleep apnea presented to the emergency department for generalized weakness and intermittent confusion gradually worsening over two months, as described by her family. On physical examination, she was oriented only to person. She stated she was in a library and when told she was at the hospital, she did not understand why. She had no complaints at the time. Her gait was ataxic, favoring her right, and she exhibited bidirectional nystagmus.
Stroke, specifically involving the posterior circulation, was considered; however, it was much lower on the list of differentials. Initial workup was notable for a slightly low potassium of 3.3 mmol/L in the setting of hydrochlorothiazide use. The patient’s CMP and CBC were otherwise unremarkable. HIV, RPR, TSH, and ammonia testing were all normal. A head CT demonstrated no acute findings to explain her symptoms.
The patient was admitted for altered mental status. Neurology was consulted and recommended an MRI and EEG. The MRI, performed while the patient was boarding in the ED, was normal. The EEG demonstrated nonspecific moderate diffuse background slowing indicative of diffuse cerebral dysfunction seen with various etiologies of encephalopathy.
Further review of records and discussion with the patient’s family showed that she had lost approximately 70 pounds in the past three months since starting semaglutide, a prescription weight loss medication. The medication had significantly impacted her appetite. In addition, four days prior, she had presented to an outside ED for concerns by family that she was having trouble walking and seemed confused. She was discharged from that ED given reassuring vitals, a non-focal exam, and a normal head CT. Two weeks prior, she was seen at the same ED twice in one week, both times for GI complaints. During the first visit, she had an abdominal CT demonstrating diverticulitis. She was discharged at that time on oral antibiotics and, after the second visit, a proton pump inhibitor.
Given this history, with the triad of altered mental status, bidirectional nystagmus, and ataxia, a presumptive diagnosis of WE was made, and high-dose IV thiamine was started. After a single high dose (500mg) was given, thiamine was not continued by the admitting team, as the patient did not appear malnourished and lacked a history of alcohol abuse.
Discussion
WE is a frequently missed diagnosis. In part, this is because the classic triad of confusion, gait or truncal ataxia, and eye movement abnormalities are present less than 20% of the time. The confusion may present as only a subtle “mental sluggishness,” and eye movement abnormalities may present as either nystagmus or ophthalmoplegia.2
Subclinical thiamine deficiency is incredibly difficult to diagnose, as only nonspecific symptoms such as headache, fatigue, irritability, and abdominal discomfort are present. It may be impossible to make the diagnosis in an intoxicated, alcoholic patient.3 The diagnosis is more likely to be missed in patients without a history of alcohol abuse. In one study over five years, 52 cases of WE were discovered on autopsy, with only four diagnosed clinically beforehand — all in patients with alcohol use disorders, while 23% of cases were in those without.4
With the trends of slowly increasing alcohol consumption among Americans in the past 30 years and a 100,000-fold increase in bariatric surgery over a decade, we can expect the rate of WE to increase.5-6
Bariatric surgery is an increasingly recognized risk factor for WE. Other risk factors to consider include anorexia nervosa, hyperemesis gravidarum, and prolonged parenteral feeding, as well as AIDS, malignancy, ESRD, and heart failure, particularly with diuretic use.7
Our patient is suspected to have developed WE in the setting of prominent weight loss since starting semaglutide. Further thiamine deficiency was likely triggered by her GI illness in the weeks prior to presentation.
Semaglutide is a once-weekly injectable glucagon-like peptide-1 receptor (GLP-1) agonist, a class initially developed for type-2 diabetes. This class is now a first-line option for both type-2 diabetes and for inducing weight loss in patients with obesity. The most common side effects are mild nausea and diarrhea, which tend to improve over time.8 Sold under the brand names Wegovy and Ozempic, these drugs have become increasingly popular after celebrities claimed their use on social media.
There are case reports of patients receiving the diagnosis of WE in the setting of restrictive diets and non-FDA-approved supplement use.9 However, this is the first case of WE attributed to prescription weight loss medication that we know of in the literature.
Typically, lab test results for thiamine are delayed and MRI is not readily available to the emergency physician, nor is it particularly sensitive for the disease.3 Therefore, the diagnosis is clinical and should be made if the patient has two of the following: dietary deficiency, oculomotor abnormality, cerebellar dysfunction, and either altered mental status or mild memory impairment.10 Eventually, low blood thiamine levels — as well a bilateral, symmetrically increased T2 signal on MRI involving the mamillary bodies, hypothalamus, or paraventricular thalamus — support the diagnosis.3
Delays in treatment or under-dosing parenteral thiamine may result in coma and death. Therefore, if suspected, empiric treatment should be initiated with high-dose IV thiamine therapy, 500mg every eight hours for three days.11Glucose before thiamine may precipitate WE, but do not withhold glucose for the unstable hypoglycemic patient while ordering thiamine.7 Magnesium deficiency should be treated to prevent refractory encephalopathy, and expect concurrent deficiencies of vitamins B6, B12, and folate.12
With parenteral treatment, oculomotor changes are expected to resolve quickly, within days. In fact, if you do not see oculomotor improvements, the diagnosis is unlikely and supplementation may be stopped. Variable degrees of ataxia and cognitive impairment may persist permanently.13
Case Conclusion
While the patient was boarding in our ED, a second opinion on the MRI was requested specifically to evaluate for mamillary body changes, and our neuroradiologist noted enhancement around the mamillary bodies consistent with WE (Figure 1).
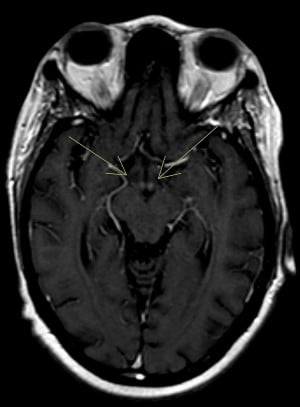
Figure 1: Post-contrast axial view demonstrating increased signal intensity involving the mamillary bodies
A thiamine level was sent, which ultimately resulted low. B12 and folate levels also returned low. The patient was restarted on high-dose IV thiamine, and by day two, her horizontal nystagmus had resolved. Her confusion persisted for more than a week, but ultimately, she was discharged to a skilled nursing facility for rehab with improved cognition. At neurology follow-up two months later, there was no comment of visual or gait disturbances. The patient is experiencing ongoing issues with short-term memory but is otherwise doing well at home.
References
- Sullivan, E. V., & Fama, R. (2012). Wernicke’s encephalopathy and Korsakoff’s syndrome revisited. Neuropsychology Review, 22(2), 69–71. https://doi.org/10.1007/s11065-012-9205-2
- Bilici, R., Saridogan, G., Turan, C., Goncu, T., Akdur, O., Citak, S., & Domac, F. M. (2015). A case of Wernicke-Korsakoff syndrome treated 1 year after the onset of symptoms. The Primary Care Companion For CNS Disorders, 17(6). https://doi.org/10.4088/pcc.15l01801
- Sechi, G. P., & Serra, A. (2007). Wernicke's encephalopathy: New clinical settings and recent advances in diagnosis and management. The Lancet Neurology, 6(5), 442–455. https://doi.org/10.1016/s1474-4422(07)70104-7
- Lindboe, C. F., & Løberg, E. M. (1989). Wernicke's encephalopathy in non-alcoholics. Journal of the Neurological Sciences, 90(2), 125–129. https://doi.org/10.1016/0022-510x(89)90095-6
- Ritchie, H., & Roser, M. (2018, April 16). Alcohol consumption. Our World in Data. Retrieved October 21, 2022, from https://ourworldindata.org/alcohol-consumption
- American Society for Metabolic and Bariatric Surgery. (2022, June 27). Estimate of bariatric surgery numbers, 2011-2020. Retrieved October 21, 2022, from https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- Reyes, J. (2021, August 19). Thiamine deficiency: Pearls and pitfalls. emDOCs.net - Emergency Medicine Education. Retrieved October 20, 2022, from http://www.emdocs.net/thiamine-deficiency-pearls-pitfalls/
- Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M. T. D., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., & Kushner, R. F. (2021, March 18). Once-weekly semaglutide in adults with overweight or obesity: Nejm. Retrieved October 28, 2022, from https://www.nejm.org/doi/full/10.1056/NEJMoa2032183
- Tóth, A., Aradi, G., Várallyay, G., Arányi, Z., Bereczki, D., & Vastagh, I. (2014). Wernicke’s encephalopathy induced by the use of diet pills and unbalanced diet. Orvosi Hetilap, 155(12), 469–474. https://doi.org/10.1556/oh.2014.29847
- Galvin, R., Bråthen, G., Ivashynka, A., Hillbom, M., Tanasescu, R., & Leone, M. A. (2010). EFNS guidelines for diagnosis, therapy and Prevention of Wernicke Encephalopathy. European Journal of Neurology, 17(12), 1408–1418. https://doi.org/10.1111/j.1468-1331.2010.03153.x
- Thomson, A., Guerrini, I., & Marshall, E. (2012). The evolution and treatment of Korsakoff's syndrome. Neuropsychology Review, 22(2), 81–92. https://doi.org/10.1007/s11065-012-9196-z
- Thomson, A. D., Cook, C., Touquet, R., & Henry, J. (2002). The Royal College of Physicians Report on Alcohol: Guidelines for managing Wernicke's encephalopathy in the accident and emergency department. Alcohol and Alcoholism, 37(6), 513–521. https://doi.org/10.1093/alcalc/37.6.513
- Donnino, M., Vega, J., Miller, J., & Walsh, M. (2007). Myths and misconceptions of Wernicke’s encephalopathy: What every emergency physician should know. Annals of Emergency Medicine, 50(6), 715–721. https://doi.org/10.1016/j.annemergmed.2007.02.007