An 81-year-old man with a past medical history of multiple deep venous thrombosis (DVT), pulmonary embolism (PE), and paroxysmal atrial fibrillation presented to the emergency department with an acute onset bilateral arm rash.
Prior hypercoagulable workup was negative for factor V Leiden mutation, prothrombin gene mutation, protein C and S deficiency, and antiphospholipid syndrome.
The patient was recently transitioned to oral anticoagulation warfarin from subcutaneous weight-based enoxaparin. The patient sought treatment two hours after performing yard work. He noticed a rash spreading on his left forearm with pain and paresthesia. He denied pruritus, trauma to the area, or prior history of rashes or allergic reactions. The patient admitted to holding a pesticide canister in the affected limb while he was spraying shrubbery.
His clotting history was significant for a provoked DVT with subsequent PE in 2006, requiring three months of anticoagulation with warfarin without any complications. The patient had a second unprovoked DVT in 2011 and was placed on lifelong anticoagulation with warfarin. In 2019, anticoagulation was switched from warfarin to a direct oral anticoagulant with apixaban due to new onset paroxysmal atrial fibrillation.
In May 2022, the patient developed an unprovoked DVT of his left femoral vein with complete occlusion of the mid segment while on apixaban. The patient was transitioned to weight-based enoxaparin for anticoagulation. Due to injection site discomfort with enoxaparin, the patient opted to transition back to warfarin for anticoagulation with an enoxaparin bridge.
Physical exam was pertinent for purpura with ulceration and blistering on the dorsum aspects of the forearms bilaterally. The left forearm demonstrated a greater girth and more profound rash. No bullae were appreciated (Image 1). Basic laboratory workup was significant for a therapeutic INR of 2.8, a baseline hemoglobin level of 11.7 mg/dL, and platelets of 215x109/L. X-rays demonstrated no evidence of fracture or subcutaneous soft tissue calcium deposits.
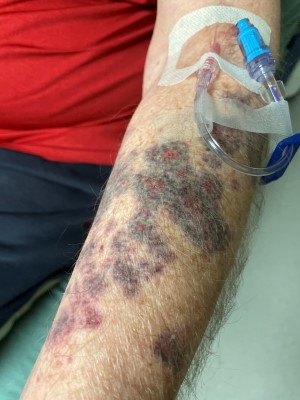
Image 1. Purpuric rash of the left forearm with several annular ecchymoses
As the rash was not characteristic of traumatic ecchymosis, hematology/oncology was consulted for suspected warfarin-induced skin necrosis (WISN).
Hematology/oncology recommended additional diagnostics with a DVT ultrasound of all extremities and a baseline protein C activity. The DVT ultrasound of upper extremities was negative for any thrombi, and the DVT ultrasound of lower extremities showed a partial occlusion of the left femoral vein, which was mildly improved from May 2022. Intravenous vitamin K 10 mg and 2 units of fresh frozen plasma (FFP) were administered, and continuous intravenous heparin was started prior to hospital admission.
Discussion
WISN is a rare complication occurring in 0.01-0.1% of patients taking warfarin, especially at higher doses.1 It is most commonly seen in middle-aged women, occurring on the breast, buttocks, and thighs, but can be present in any location.1 It most commonly occurs within 5-10 days, as it takes warfarin several days to have full effect.2 The pathogenesis is believed to be related to the reduction of vitamin-K dependent anticoagulant factors, and proteins C and S. Vitamin-K dependent clotting factors II, VII, IX, and X are also reduced, but proteins C and S have significantly shorter half-lives compared to clotting factors II, VII, IX, and X.2 This decrease in proteins C and S creates a paradoxical hypercoagulable state, which leads to thrombotic occlusion of the microvasculature and resulting necrosis.2Patients started on warfarin are frequently bridged with a heparin product for at least five days of therapy or until therapeutic INR goal is achieved to prevent WISN.3
Diagnosis
Warfarin-induced skin necrosis is a clinical diagnosis but is supported by predisposing hypercoagulable diseases to include protein C and S deficiency, antiphospholipid antibody syndrome, and antithrombin gene mutation.4 The rash characteristically starts as pain and paresthesia with an initial central erythematous macule which, as edema develops, progresses to a central purpuric zone, then finally necrosis.4
Lab work can be beneficial for further management of the patient outside of the ED and is necessary to evaluate for other pathologies that can mimic WISN. The differential includes hematoma, disseminated intravascular coagulation, purpura fulminans, cellulitis, necrotizing fasciitis, and calciphylaxis.
The patient was treated for WISN and started on heparin-based therapy to prevent further microvascular thrombosis. He did well inpatient with improvement of his lesions while on heparin and was transitioned to subcutaneous enoxaparin. His lab work returned a protein C activity assay of 45%, which favors acquired protein C deficiency, likely secondary to diet lacking in vitamin K. Acquired protein C deficiency can also be seen in DIC, infection, and hepatic dysfunction; further investigation through alternative testing modalities is necessary for confirmation.5
Given suspicion of protein C deficiency, the patient was advised to complete a prolonged bridge with weight-based enoxaparin prior to transitioning back to a lower dose of warfarin.
Management
WISN management involves reversing warfarin and starting the patient on a heparin product to prevent further microvascular thrombi.3 In this case, warfarin was reversed utilizing 10 mg of vitamin K and two units of FFP.3 Vitamin K is used to provide enough substrate to overcome the inhibition of vitamin K epoxidase inhibition by warfarin. FFP contains all of the vitamin K-dependent coagulation factors for reversal of coagulopathy.6, 7
Other reversal options for patients with fluid overload include 4-factor prothrombin complex concentrate (PCC), which has purified concentrates of factors II, VII, IX, and X for reversal of coagulapathy.6,7 However, PCC can contain varying protein S and C concentrates depending on the manufacturer.7
Case Conclusion
The patient was concomitantly transitioned to an unfractionated heparin drip. Low molecular weight heparin is another reasonable option; however, we opted for a titratable option in the event of complications for this patient who required admission.
Conclusion
Rash and skin lesion differentials are vast and often subtle. Varying clinical scenarios present a diagnostic challenge for emergency physicians at any stage of their career. Warfarin-induced skin necrosis is a rare condition thought to be due to microvascular thrombosis and ischemia and is commonly related to protein C deficiency. Recognition of WISN is critical to reduce morbidity and mortality. The level of protein C is not necessary for diagnosis. Principles of management include reversing warfarin therapy with vitamin K and FFP as well as starting the patient on therapeutic heparin with hematology/oncology consultation.
References
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. British Journal of Surgery. 2000;87(3):266-272.
- Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin-induced skin necrosis. Annals of Dermatology. 2014;26(1):96-98.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease. Chest. 2012;141(2):1698-1704.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. Journal of the American Academy of Dermatology. 2009;61(2):325-332.
- Khor B, Van Cott EM. Laboratory tests for protein C deficiency. American Journal of Hematology. 2010;85(6):440-442.
- Hanley JP. Warfarin reversal. Journal of Clinical Pathology. 2004;57(11):1132-1139.
- Eichinger S. Reversing vitamin K antagonists: making the old new again. Hematology Am Soc Hematol Educ Program. 2016;2016(1):605-611.