Can you recognize severe valproate overdose? Understanding VPA pharmacokinetics is essential to help guide your treatment plan.
A 53-year-old man with a history of bipolar affective disorder presents to the ED after a suicidal ingestion of approximately 30 tablets of valproic acid and 14 tablets of haloperidol. He is hemodynamically stable and somnolent, but easily arousable and able to answer questions appropriately. He has no signs or symptoms of anticholinergic toxicity or extrapyramidal symptoms.
Background
Most physicians are well acquainted with the teratogenic potential of valproic acid (VPA) and have an intuitive understanding of its hepatotoxic potential. For the emergency physician, many cases of acute VPA overdose result in only mild impairment. However, recognition of severe toxicity is essential, and an understanding of VPA pharmacokinetics can help guide treatment.1
Pharmacology and Pharmacokinetics
VPA is a widely prescribed anticonvulsant and mood stabilizer that is also used for migraine prophylaxis and treatment of neuropathic pain.2, 3 The described mechanism of action accounting for its anticonvulsant activity is prolonged inactivation of neuronal voltage-activated sodium channels similar to the action of carbamazepine and phenytoin.2 Additionally, inhibition of GABA metabolism has been shown to increase this inhibitory neurotransmitter in vitro though the relationship of this effect to in vivo anticonvulsant activity is unclear. Oral absorption of VPA is rapid and complete, peak levels are reached 1-4 hours after ingestion, and the elimination half-life is approximately 15 hours. At therapeutic concentrations (50-100 mcg/dL), it is highly protein bound (90%) with an apparent volume of distribution of approximately 0.2 L/kg.2 Hepatic metabolism via UDP-glucuronosyltransferases and beta-oxidation predominates. Toxicity CNS depression is common, ranging from somnolence and lethargy to severe coma.3, 4 Respiratory depression, hypotension, metabolic acidosis, and electrolyte abnormalities (such as hypocalcemia, hypernatremia, and hypophosphatemia) are possible.3 Unique features of acute overdose include hepatitis, pancreatitis, thrombocytopenia, and leukopenia.5 Delayed symptoms and peak concentrations should be anticipated in cases of enteric coated formulations.6, 7
Management and Treatment
For the patient with a known or suspected ingestion, serum ammonia and VPA concentrations should be obtained in addition to liver function tests. A 4-hour post-ingestion acetaminophen concentration should be obtained in all patients with intentional ingestions. Due to the delayed absorption of enteric-coated formulations, VPA and ammonia concentrations should be followed until they begin to downtrend.3
Supportive Measures
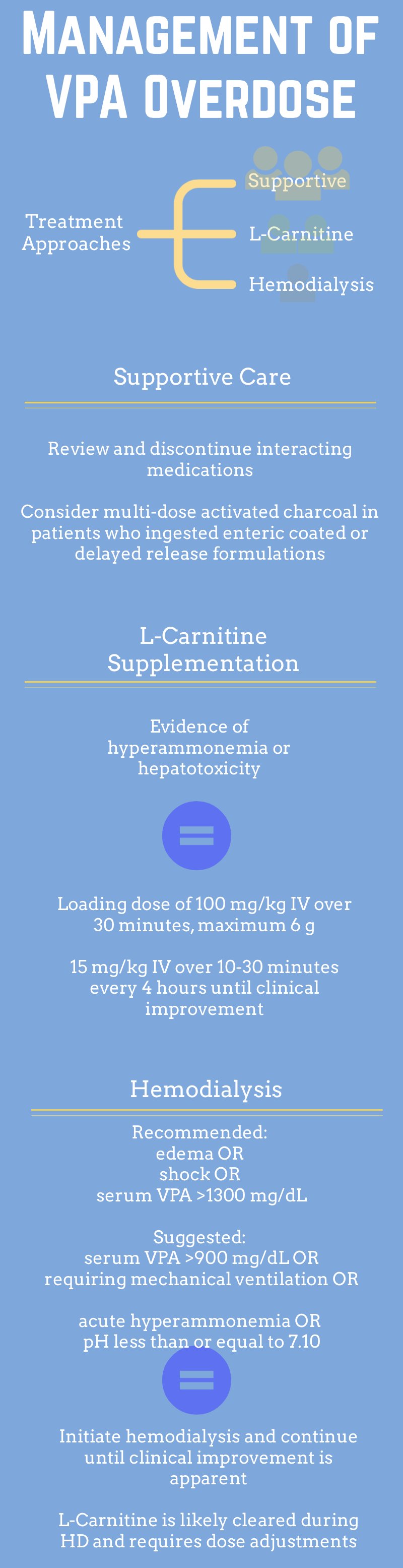
In addition to following serum drug concentrations, it is reasonable to consider multi-dose activated charcoal to reduce absorption in ingestions of enteric coated formulations. It is also recommended to review and discontinue medications which affect VPA metabolism.3
L-carnitine Supplementation
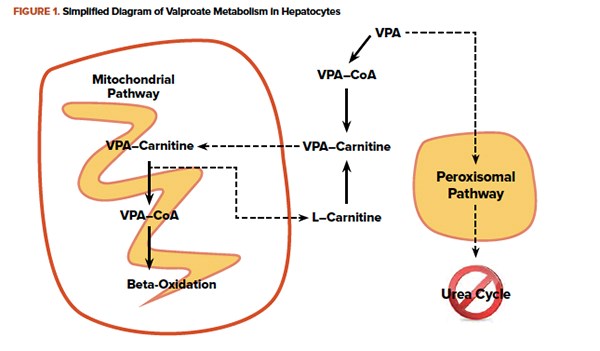
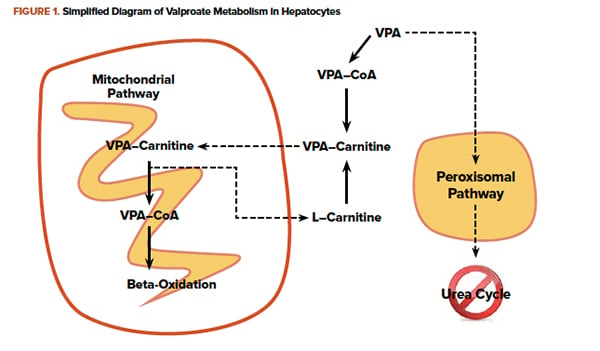
Within hepatocytes, VPA is metabolized as a fatty acid and is shuttled into mitochondria conjugated to carnitine. In overdose, the quantity of VPA overwhelms mitochondrial beta-oxidation. VPA and other fatty acids accumulate in the hepatocytes causing steatosis. Additionally, VPA in the cytoplasm is metabolized by an alternate oxidation pathway. A byproduct is an inhibitor of the urea cycle ultimately raising serum ammonia concentrations. Administration of L-carnitine enhances movement of VPA into the mitochondria, where it will eventually be metabolized.8 Clinical studies currently consist only of retrospective case reports and case series, but good clinical outcomes without adverse events are described.9, 10 Figure 1 briefly outlines metabolic pathways, and dosing recommendations are outlined in Figure 2.
Hemodialysis
Typical VPA pharmacokinetics would preclude hemodialysis due to the high degree of protein binding. However, at extremely high serum concentrations the protein bound fraction decreases and provides a rationale for hemodialysis.4 For patients with manifestations of cerebral edema or shock, or serum VPA concentration 1300 mg/dL, intermittent hemodialysis is recommended.11 Other considerations and treatment parameters are outlined in Figure 2.
Emerging Approaches
A recently published case series (n=5) proposes L-arginine supplementation as an additional therapeutic approach in VPA overdose patients to stimulate N-acetylglutamate synthetase, which is inhibited by VPA.12 Although the case series shows a temporal relationship between declining ammonia concentrations and L-arginine supplementation, 2 of the 5 patients were also treated with hemodialysis, and further study of larger cohorts need to be undertaken.
Case Resolution
The patient had an initially elevated VPA concentration that peaked 8 hours post-ingestion at 437 mg/dL. Prior to starting L-carnitine, this patient also had a peak ammonia level of 133 micromol/L. Liver transaminases and INR in the ED were within normal limits. The patient was admitted to the MICU and L-carnitine was continued for 48 hours. Subsequently deemed medically stable, the patient was transferred to inpatient psychiatry.
References
1. Isbister GK, Balit CR, Whyte IM, Dawson A. Valproate overdose: a comparative cohort study of self poisonings. Br J Clin Pharmacol. 2003;55(4):398-404.
2. Goodman LS, Gilman A, Brunton LL. Goodman & Gilman's manual of pharmacology and therapeutics. New York, NY: McGraw-Hill Medical; 2008.
3. Hoffman RS, Howland MA, Lewin NA, Nelson L, Goldfrank LR, Flomenbaum N. Goldfrank's toxicologic emergencies. 10th ed. New York, NY: McGraw-Hill Education; 2015.
4. Tintinalli JE, Stapczynski JS, Ma OJ, Cline D, Meckler GD, Yealy DM. Tintinalli's emergency medicine : a comprehensive study guide. 8th ed. New York, NY: McGraw-Hill Education; 2016.
5. Spiller HA, Krenzelok EP, Klein-Schwartz W, et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38(7):755-760.
6. Graudins A, Aaron CK. Delayed peak serum valproic acid in massive divalproex overdose--treatment with charcoal hemoperfusion. J Toxicol Clin Toxicol. 1996;34(3):335-341.
7. Ingels M, Beauchamp J, Clark RF, Williams SR. Delayed valproic acid toxicity: a retrospective case series. Ann Emerg Med. 2002;39(6):616-621.
8. Lheureux PE, Penaloza A, Zahir S, Gris M. Science review: carnitine in the treatment of valproic acid-induced toxicity - what is the evidence? Crit Care. 2005;9(5):431-440.
9. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
10. Glatstein M, Bonifacio Rino P, de Pinho S, Scolnik D, Pivko-Levi D, Hoyte C. Levocarnitine for the Treatment of Valproic Acid-Induced Hyperammonemic Encephalopathy in Children: The Experience of Large, Tertiary Care Pediatric Hospital and a Poison Center. Am J Ther. 2017. Epub ahead of print; doi 10.1097/MJT.0000000000000706.
11. Ghannoum M, Laliberte M, Nolin TD, et al. Extracorporeal treatment for valproic acid poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila). 2015;53(5):454-465.
12. Schrettl V, Felgenhauer N, Rabe C, Fernando M, Eyer F. L-Arginine in the treatment of valproate overdose - five clinical cases. Clin Toxicol (Phila). 2017;55(4):260-266.