An 85-year-old female with a history of hypertension and chronic obstructive pulmonary disease (COPD) presents with sudden onset of dyspnea on exertion. She was taken off of warfarin three months ago for a prior PE that had occurred two years ago. She currently denies any chest pain, nausea, vomiting, hemoptysis, fevers, or syncope. Vital signs show a blood pressure of 190/90, a heart rate at 95, a respiratory rate of 18, and an oxygen saturation of 93% on 4L nasal cannula, though she is not in any respiratory distress. Other than mild bilateral pitting edema, her exam is normal. Labs are sent, and pending. Her differential is still quite broad; how can focused bedside thoracic and cardiovascular ultrasound assist in making a diagnosis?
Thoracic Ultrasound
Most providers are aware that thoracic ultrasound can assess for presence of a pneumothorax using the high frequency probe over a non-dependent intercostal space on the anterior chest wall. Here in normal lung pleural sliding can be seen, sometimes called “ants marching”. Absence of pleural sliding is the most sensitive finding for pneumothorax. Similarly, it can examine the “seashore sign” on M-mode imaging to exclude the diagnosis, or witness a “barcode or stratosphere sign” to confirm the presence of a pneumothorax. The specificity can range from 60-99%.1 However, the negative predictive value for lung sliding is 99.2-100%, which indicates that if lung sliding is present then pneumothorax is ruled out in the plane where sliding is visualized.1 Multiple views in each hemithorax must be used to completely evaluate for absence of a pneumothorax.
Pulmonary edema resulting from decompensated heart failure or other fluid-overloaded states can be detected using the low frequency probe over multiple bilateral intercostal spaces, looking for the presence of B-lines. The B-lines are comet tail artifacts that are created when air and fluid interface at the alveolar interstitial membrane in the lungs. These B-lines are considered pathological when greater than three lines are present in a given interspace, and are suggestive of interstitial edema. This finding touts a sensitivity of 97%, and specificity of 95%.2 Both evaluation of pneumothorax and pulmonary edema are useful in the workup of acute dyspnea.
Focused Cardiac Ultrasound
A focused cardiac ultrasound can also be used for the workup and evaluation of dyspnea and PE. A low frequency phased array transducer can assess for pericardial effusion and evaluate the left ventriclular (LV) systolic function. All windows (parasternal long and short, apical, and subcostal) should be utilized. The apical four chamber view will allow the clinician to evaluate the right and left heart side-by-side and will also permit use of color Doppler to assess for any abnormalities of the atrioventricular valves (tricuspid and mitral).
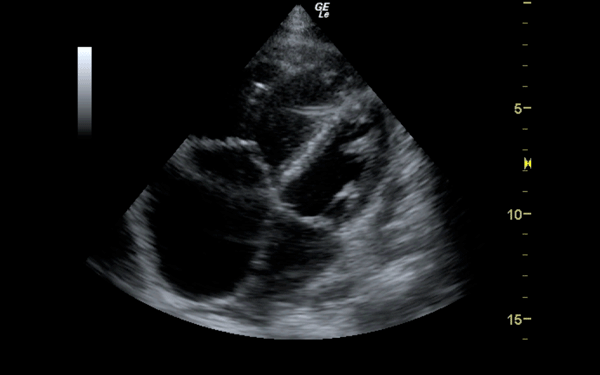
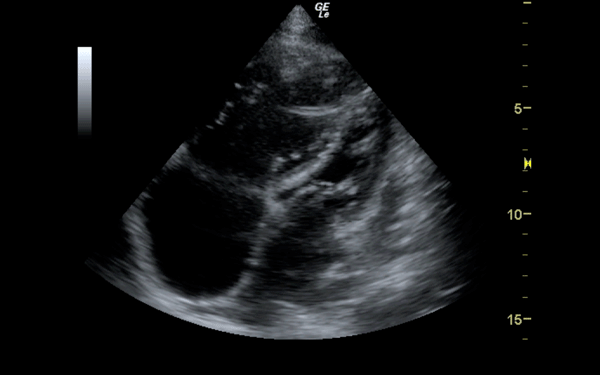
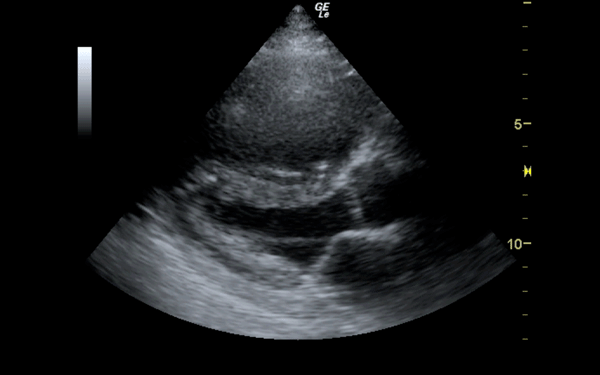
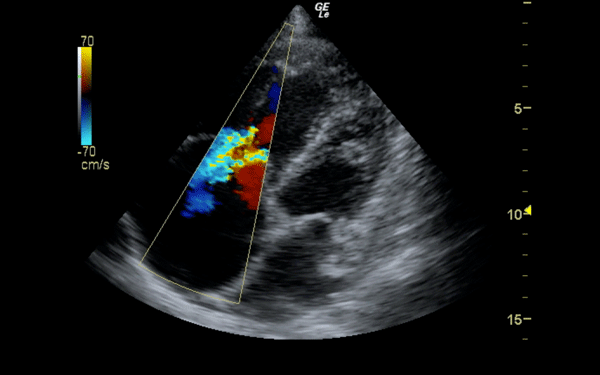
If there is a pretest concern for PE, or if the diagnosis has already been established, you may be able to see signs of right heart strain on cardiac ultrasound. Dilation of the right ventricle (RV) and hypokinesis of the free wall are the most likely findings to suggest RV strain. Examples of RV dilation and strain in both systole and diastole are demonstrated in Figures 1 and 2. McConnell's sign consists of right ventricular dilation and hypokinesis with sparing of the apex and is a distinct echocardiographic finding in acute PE. This is most often seen in massive PEs. This sign has low sensitivity but up to 94% specificity for the diagnosis of PE.3,4 The presence of paradoxical septal motion, which is expressed as bowing of the interventricular septum into the LV outflow tract, is best observed in the parasternal short view, but can also be seen in the long axis (Figure 3). Occasionally, the severity of RV dilation can lead to valvular dysfunction, usually tricuspid regurgitation (Figure 4). Overall, the echocardiogram is a useful tool in patients with moderate to high risk for PEs. Findings of right heart strain have been shown in multiple studies to predict an increase in in-hospital mortality.
Pulmonary Embolism: Risk Stratification
PE severity is based on the extent of pulmonary artery occlusion and the underlying cardiopulmonary reserve of the patient. Patients with massive PEs have hemodynamic instability and are often treated with systemic tissue-plasminogen activator (t-PA), catheter-directed t-PA, or surgical thrombectomy. There are 150,000 cases of PE diagnosed annually; most of these patients are normotensive (SBP >90 mmHg), and only a small population have massive PEs.5-7 Of the patients diagnosed with PE, 3% die within 48 hours, 10-15% die of PE within 30 days, and 40% of the survivors develop RV systolic dysfunction or pulmonary hypertension.5-7
When considering prognostic markers in acute PE, RV dysfunction is more predictive of clinical deterioration than other markers. The most common cause of death within 30 days is RV failure.8 The importance of RV dysfunction for normotensive patients with PE was evaluated by Kucher, et al. They selected patients from the International Cooperative Pulmonary Embolism Registry (ICOPER) and investigated those patients who had echocardiograms performed within 24 hours of their diagnosis. In their study, normotensive patients with RV systolic hypokinesis were compared to patients with normal RV function to assess the 30-day survival rate.
At 30 days the survival rate in patients with RV hypokinesis was 83.7% (95% CI, 79.3%-87.0%), and for those without RV hypokinesis it was 90.6% (95% CI 88.0%-92.6%). After adjusting for other predictors of mortality including cancer, heart failure, COPD, age greater than 70, and systolic blood pressure less than 100 mm Hg, RV hypokinesis remained an independent predictor of 30-day mortality (hazard ratio 1.94, 95% CI 1.23-3.06).8
Echocardiography in conjunction with findings of right ventriclular strain on ECG was evaluated in a prospective study of 386 normotensive patients performed by Vanni, et al. Echocardiographic evidence of right ventricle strain was defined as a right bundle branch block, S1Q3T3 pattern, or a T-wave inversion in leads V1-4. Echocardiograhic RV dysfunction was defined as paradoxical septal systolic motion, end-diastolic diameter >30 mm or RV/LV ratio >1, or pulmonary hypertension with RV/RA gradient greater than 30 mmHg. They found that patients with either RV strain on ECG or echocardiographic evidence of RV dysfunction had an increased risk of in-hospital clinical deterioration or death. However, those patients with RV strain on both echocardiography and ECG had the highest incidence of death or deterioration (hazard ratio 8.47, 95% CI 2.43-29.47).9 Patients without either of these findings had a less than 2% incidence of in-hospital death or deterioration, indicating that clinicians can use a combination of ECG and echocardiography to identify a low-risk group of normotensive patients with acute PE.
Ultrasound and ECG findings should also be interpreted in conjunction with laboratory tests typically performed for evaluation of PE (brain natriuretic peptide and troponin).10 Kline, et al. investigated the use of a test panel composed of ECG, pulse-ox, and troponin, and compared it to RV dysfunction on echocardiogram.11 The study was used to explore the utility of the proposed prognostic panel in cases where prompt availability of echocardiogram is lacking. This study revealed that the panel had prognostic equivalence to echocardiogram in terms of predicting adverse outcomes at 6 months for normotensive patients with acute PE.11 This further reinforces the utilization of a panel of tests in addition to echocardiography as a means of risk stratifying patients suffering from acute PE.
Case Conclusion
Our patient's labs reveal an elevated troponin, NT pro-BNP, and D-dimer. Her ECG has no ischemic changes. A focused cardiac ultrasound reveals a dilated, hypokinetic RV with sparing of the apex ”“ a positive McConnell's sign. Her IVC is dilated and plethoric, suggestive of extremely high right atrial pressures. CT scan reveals extensive bilateral pulmonary emboli and evidence of right heart strain. She is started on heparin and admitted to the MICU for further monitoring, and after several days she is discharged home after being restarted on oral anticoagulation.
References
- Hussain LF, Hagopian L, Wayman D et al: Sonographic Diagnosis of Pneumothorax. Journal of Emergencies, Trauma and Shock 5(1):76-81, 2012.
- Sperandeo M, Rotondo A, Guglielmi G, et al: Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: Use and Limitations. Chest Radiology 2014.
- McConnell MV, Solomon SD, Rayan ME, et al: Regional Right Ventricular Dysfunction Detected by Echocardiography in Acute Pulmonary Embolism. American Journal of Cardiology 78:469-473, 1996.
- Goldhaber SZ. Echocardiography in the Management of Pulmonary Embolism. Annals of Internal Medicine 136(9):691-700¸ 2002.
- Stein PD, Hull RD, Ghali WA, et al: Tracking the uptake of evidence: Two decades of hospital practice trends for diagnosing deep vein thrombosis and pulmonary embolism. Arch Intern Med 163:1213-1219, 2003.
- Goldhaber SZ, Visani L, De Rosa M: Acute pulmonary embolism: Clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER). Lancet 353:1386-1389, 1999.
- Ribeiro A, Lindmarker P, Johnsson H, et al: Pulmonary embolism: One-year follow-up with echocardiography Doppler and five-year survival analysis. Circulation 99:1325-1330, 1999.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ, et al: Prognostic Role of Echocardiography Among Patients With Acute Pulmonary Embolism and a Systolic Arterial Pressure of 90mmHg or Higher. Arch Intern Med 165:1777-1781, 2005.
- Vanni S, Polidori G, Vergara R, et al: Prognostic Value of ECG Among Patients with Acute Pulmonary Embolism and Normal Blood Pressure. The American Journal of Medicine 122(3):257-264, 2009.
- Kucher N, Printzen G, Goldhaber SZ, et al: Prognostic Role of Brain Natriuretic Peptide in Acute Pulmonary Embolism. Circulation 107:2545-2547, 2003.
- Kline JA, Hernandez-Nino J, Rose GA. Surrogate Markers for Adverse Outcomes in Normotensive Patients with Pulmonary Embolism. Critical Care Med 34(11):2773-2780, 2006.