Second-generation antipsychotics (SGA), including olanzapine, are effective and appropriate treatments for acute agitation and psychosis in the emergency department.1
SGAs have increasingly replaced first-generation antipsychotics like haloperidol due to their safer side effect profile.2 Among the antipsychotics, olanzapine has shown to have minimal QTc-prolonging effects.3 Nevertheless, this case demonstrates that there is still a risk of QTc prolongation and torsades de pointes (TdP) with olanzapine use in the emergency setting.
Case
A 39-year-old female with a past medical history of diabetes mellitus type II, hypertension, hyperlipidemia, chronic kidney disease, right parietal watershed stroke presented with altered mental status. Per her family, last night, the patient was vomiting and had elevated finger stick blood glucose levels at home. The patient’s husband went to work this morning and was unable to contact her throughout the day, so he called 911. EMS found the patient lying on the floor. The patient denies pain but is confused, only saying “yeah” or “no” to most questions. Per EMS, the patient’s finger stick blood glucose read “high.” Her most recent documented medication use included only methocarbamol and tramadol from an emergency department visit after a motor vehicle crash.
On arrival in the ED, her vital signs were significant for hypertension to 212/107, but were otherwise normal. On exam, she was obese, awake, not following commands, not conversational, but answering some yes or no questions appropriately. Her pupils were equal and reactive, she was moving all extremities, and her neck was supple. However, she did have mild tenderness to palpation to the lower abdomen without rigidity, rebound, guarding, or costovertebral tenderness bilaterally. Labetalol 10 mg IV was also ordered.
Initial labs and imaging showed a sodium of 134 mmol/L, potassium of 3.3 mmol/L, chloride 95 mmol/L, serum creatinine 2.2 mg/dL, glucose of 561 mg/dL, CO2 23 mmol/L, anion gap 19, albumin 2.8 g/dL, white blood cell count 18.66 x109/L, beta-hydroxybutyrate 0.3 mmol/L, urine drug screen positive for oxycodone, a urinalysis with no signs of infection, chest X-ray with only hypoinflated lungs without infiltrates, and pH of 7.43 as well as lactic acid 2.3 mmol/L on venous blood gas.
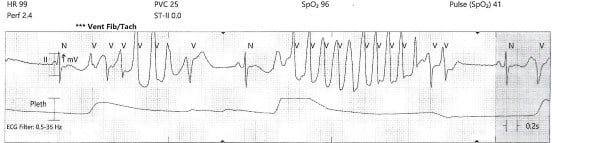
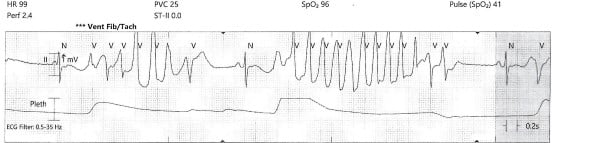
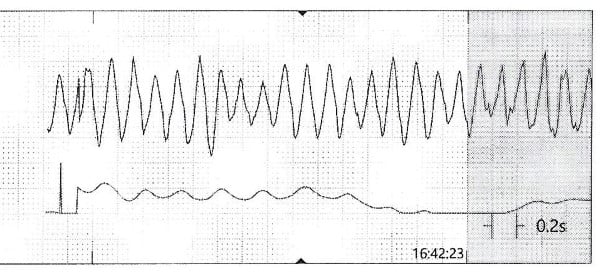
While the workup was pending, the patient became agitated and poorly redirectable, then started to yell and get out of bed. 10 mg of IM olanzapine was ordered and administered prior to obtaining an EKG. About 5 to 10 mins later, the patient started to have polymorphic premature ventricular contractions lasting several seconds. Before the nurse could perform a stat EKG, the patient fell unconscious without a pulse and ACLS was initiated. Multiple rhythm checks showed torsades de pointes then pulseless ventricular tachycardia requiring 4 defibrillations. The patient was given 4 mg of IV magnesium sulfate, 3 doses of epinephrine, 1 ampule of bicarbonate, 150 mg IV amiodarone. She was intubated quickly and eventually achieved return of spontaneous circulation after about 10 minutes. The initial EKG after ROSC showed normal sinus rhythm with a prolonged QTc of 513 msec, but the longest QTc seen was 687 msec about 3 hours later.
A second set of labs was ordered and drawn immediately after ROSC, with results significant for: potassium 2.6 mmol/L, glucose 457 mmol/L, creatinine 2.3 mg/dL, magnesium 2.8 mg/dL, total calcium 8.4 mg/dL, lactic acid 5 mmol/L. CT head without contrast redemonstrated her prior right MCA distribution infarct. CTA of the head and neck showed no significant abnormalities aside from large nodular left thyroid lobe. CT chest, abdomen, and pelvis with IV contrast showed left anterior rib fractures and no other significant findings. Thyroid studies later resulted with TSH mildly low at 0.11 uIU/mL, free T3 normal at 1.41 pg/mL, and free T4 normal at 1.0 ng/dL.
MRI brain several days later would show global anoxic brain injury. Repeat thyroid studies showed a TSH 0.12 uIU/mL and free T3 67 ng/dL, but T4 1.55 ng/dL consistent with sick euthyroid disease. Her hospital course was complicated by MSSA and Acinetobacter pneumonia, ESBL E. coli UTI, persistent fevers, and decubitus wounds. She was discharged nearly 3 months later after tracheostomy and PEG tube, awake, but not conversational nor following commands.
Discussion
There is only 1 published case report of torsades de pointes associated with olanzapine use in recent medical literature. Huang et. al. documented a case of torsades de pointes found by her ICD in an elderly female who had started oral olanzapine for suicidality 3 months earlier.4 She was also found to have a potassium level of 3.1 meq/L and QTc of 480 ms when hospitalized. She was switched to oral risperidone, her potassium level was repleted, and her QTc interval normalized. Hypokalemia could have been a second contributing factor to QTc prolongation, which finally led to TdP. However, she had no other reason to be hypokalemic, so the author postulates that olanzapine caused the low potassium level in this patient. Nevertheless, TdP is a rare phenomenon associated with olanzapine.
Multiple randomized control trials have also concluded that olanzapine has little to no effect on the QTc interval. Lindborg et. al. studied the effects of intramuscular olanzapine by pooling data from four different double-blinded trials of acute agitation with schizophrenia or dementia in the emergency department and found that there was no significant QTc prolongation with olanzapine use.5 Studies of oral olanzapine for psychiatric patients have demonstrated very mild increases in QTc compared to baseline.6-7 Czekella et. al. found only a clinically insignificant QTc lengthening with oral olanzapine in psychotic patients.8 Hasanain et. al. published a systematic review of second-generation antipsychotics in 2014 that also concluded that olanzapine only had “modest” effects on the QTc interval at therapeutic doses.3
This case demonstrates a likely causation between the use of olanzapine and this occurrence of torsades de pointes and QTc prolongation, but there are many other factors that could have contributed to her arrhythmia. Though the initial labs showed a slightly low level of potassium, it would not cause a prolonged QT. Unfortunately, a magnesium level was not ordered early on. After the cardiac arrest and administration of epinephrine and sodium bicarbonate, potassium dropped to 2.6 mmol/L. Though the appearance of polymorphic ventricular contractions occurred quickly, about 5 to 10 minutes after the administration of IM olanzapine, intramuscular absorption of olanzapine occurs rapidly, and 5-10 minutes is likely enough time to see adverse effects.9
Though olanzapine and other second-generation antipsychotics are known for their favorable side effect profiles, QTc prolongation and torsades de pointes are still potential complications. Therefore, be wary of administering the medication concomitantly with other QTc-prolonging medications.
If time and resources are permissible, check an EKG for any suspicion of a pre-existing prolonged QTc. If there are any indications of possible hypokalemia or hypomagnesemia, check serum electrolyte levels. For alternative chemical restraint medications, benzodiazepines and ketamine may be considered, though they carry their own risks and contraindications. Olanzapine is an extremely practical medication in the emergency physician’s toolkit, but must be used in the right setting and with vigilance for possible side effects.
References
- Moore, G. Assessment and emergency management of the acutely agitated or violent adult. In: UpToDate, Hockberger, R (Ed), UpToDate, Waltham, MA. (Accessed June 29, 2021).
- Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005 Oct;23(6):767-76.
- Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs. 2014 Oct;28(10):887-920.
- Huang G, Fu Q, Xu J. Potential torsades de pointes triggered by hypokalemia related to olanzapine in a patient with implantable cardioverter-defibrillator. J Clin Psychopharmacol. 2014 Oct;34(5):651-2.
- Lindborg SR, Beasley CM, Alaka K, Taylor CC. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res. 2003 Jul 15;119(1-2):113-23.
- Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62-9.
- Agelink MW, Majewski T, Wurthmann C, Lukas K, Ullrich H, Linka T, Klieser E. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21(1):8-13.
- Czekalla J, Beasley CM Jr, Dellva MA, Berg PH, Grundy S. Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. J Clin Psychiatry. 2001;62(3):191-8.
- Zyprexa (olanzapine) [prescribing information]. Indianapolis, IN: Lilly USA LLC; February 2021.