There are no hard and fast rules for therapeutic thoracentesis in the ED setting but there are some accepted best practices.
Case
A 65-year-old female presents to the ED for worsening shortness of breath. She has a history of limited medical care and was recently diagnosed with widely metastatic breast adenocarcinoma after discovery of pulmonary embolism for which she has remained on enoxaparin. She has a history of malignant (fluid cytology positive) pleural effusion drained two weeks prior to presentation and discharged home on 5 liters (L) of oxygen via nasal cannula (NC). Her chief complaint on presentation was worsening dyspnea on exertion over the last several days.
On arrival to the ED, she was saturating 92% on 8L NC and then placed on heated high-flow nasal cannula (HHFNC). Workup was negative for acute coronary syndrome or metabolic derangements but did show a new anemia with hemoglobin 8.4 g/dL from 11.3 g/ dL. Chest x-ray revealed large right and moderate left pleural effusions that were increased in size from prior as well as bilateral airspace opacities. She was unable to lay flat for CT and was declining intubation. She was started on empiric broad spectrum antibiotics for pneumonia and supplemental oxygen requirements continued to increase on HHFNC. The team was planning a diagnostic thoracentesis to evaluate for hemothorax given new anemia and consideration was also given to perform therapeutic thoracentesis to help improve her oxygenation.
Causes of Hypoxemia
This patient was suffering from acute on chronic hypoxemic respiratory failure. There are 5 mechanisms of hypoxemia to consider:
- Decreased inspired partial pressure of oxygen (normal A-a gradient) — Not a factor at sea level
- Hypoventilation (normal A-a gradient) — Lack of respiratory acidosis on blood gas suggested adequate minute ventilation
- Diffusion limitation (elevated A-a gradient) — Classically caused by pulmonary fibrosis with impaired gas exchange across the alveolar-capillary membrane
- Right-to-left shunt (elevated A-a gradient + inability to achieve 100% SpO2 with 100% FiO2) — Bedside echo with agitated saline showed no intracardiac shunt and CTA with contrast of the chest from month prior was without evidence of intrapulmonary shunt. Physiologic shunt was a possibility if hydrothorax causing compressive atelectasis.
- V/Q mismatch (elevated A-a gradient) — Patient had a history of PE as well as lung cancer, both of which can cause disruptions of normal V/Q ratio.
The patient was at risk for shunt and V/Q mismatch. To further investigate the presence of shunt physiology, an ABG before and after increase in supplemental oxygen (eg, nonrebreather at 15L should give roughly 80% FiO2) could have been used to evaluate the A-a gradient response. No change in the A-a gradient would suggest shunt physiology or diffusion limitation; a decrease is consistent with another cause of V/Q mismatch. Fluid in the pleural space can contribute to compression atelectasis and subsequently shunting of blood through poorly ventilated alveoli resulting in worsening V/Q matching.
Case Continued
A thoracentesis was performed with vacuum bottles which yielded 2L of bloody pleural effusion. There was subjective improvement in work of breathing and she was de-escalated from HFNC to 6L NC. Pleural fluid LDH was 127 (serum 227) and protein was 2.7 (serum 5.5). This does not meet Light's Criteria for exudative effusion; however, it does meet by Two-test and Three-test rules as LDH > 0.45 upper limit of normal.1
Several hours after thoracentesis, the patient's work of breathing again increased and a chest x-ray was performed, which revealed a small right hydropneumothorax. CT imaging was initially delayed given respiratory distress but was completed hours later and revealed large right hydropneumothorax. Thoracic Surgery was consulted and recommended small bore chest tube via Seldinger technique, which yielded 400cc of serosanguinous fluid.
The patient stabilized on 6L NC and was ultimately admitted to the floor. While on the floor, she had decreasing output from chest tube but persistent pneumothorax on chest x-ray. On day 3 of admission, she decompensated from a respiratory standpoint, requiring transfer to ICU. Pleural manometry performed in the ICU revealed low pleural pressures consistent with pneumothorax ex vacuo.
Pneumothorax Ex Vacuo
There are three types of iatrogenic pneumothorax (ie, complications post-thoracentesis) seen in the ED. The first is caused by injury to the visceral pleura by the needle or catheter/tube or from ruptured blebs in high airway pressures. The second is characterized by violation of the parietal pleura as seen during subclavian line placement, for example. The third is termed pneumothorax ex vacuo (PEV), which occurred in this case.
PEV occurs when there is a fibrous peel over the visceral pleura that prevents re-expansion even when the pleural space is drained. This can be due to lung entrapment, which describes an active process such as malignancy or infection causing acute inflammation, or trapped lung as a result of past inflammation. The other etiology of PEV is an endobronchial lesion completely occluding the bronchus preventing lung expansion despite thoracentesis. Cancer patients such as this one are at risk for this type of lesion given her widely metastatic disease.
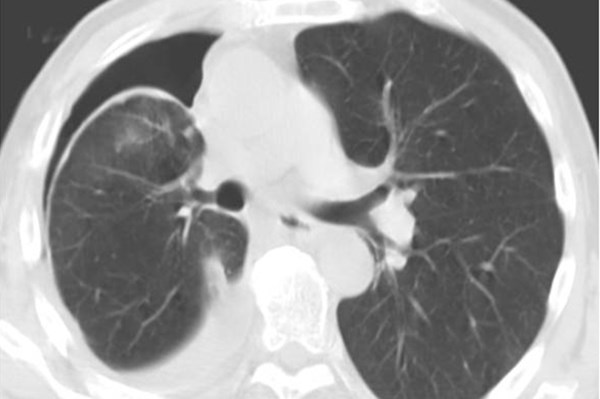
PEV differs from a typical pneumothorax because the air in the pleural space is filling a void that would otherwise be filled with fluid and not lung parenchyma. The lung parenchyma in the case of PEV cannot expand to fill the evacuated pleural space due to the restrictive fibrous peel which can sometimes be visualized on CT (Figure 1). The development of PEV can thus result in an increase in pleuritic pain for the patient without benefiting the patient's respiratory status.
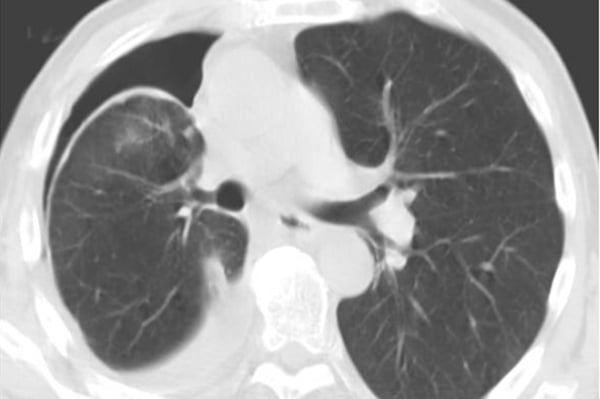
Figure 1. CT chest after therapeutic thoracentesis showing hyperdense fibrous peel over visceral pleura2
PEV should be anticipated as a complication of the drainage of chronic pleural effusions secondary to an inflammatory or malignancy-related exudative effusion where there has been time to develop a thick, fibrous ring around the lung parenchyma that limits its re-expansion. In these cases, pleural manometry should be considered.
Pleural manometry is often employed by pleural consult services and has been suggested as a tool to help prevent excessive fluid drainage as well as in the diagnosis of trapped lung. This involves measuring, with a water column or electronic manometer, the intrapleural pressure and facilitates draining to a point which can promote lung re-expansion without the complications of re-expansion edema or pneumothorax ex vacuo. The regular use of manometry in therapeutic thoracentesis has been proposed as a method to avoid complications;3 however, a validated cutoff point for pressure or volume of evacuation has not been established based on limited studies.3,4
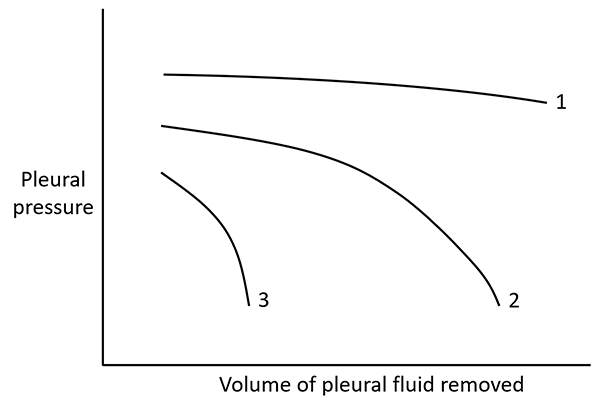
Figure 2. Pleural elastance curves. The slopes of the different curves represent pleural space elastance. Curve 1 represents a normal pleural elastance, and Curve 3 represents abnormally high pleural elastance ("trapped lung"), whereas Curve 2 represents an initial normal elastance that becomes abnormally high as more fluid is removed ("entrapped lung")5.
The techniques for fluid removal include negative pressure bottle, manual aspiration, and gravity-dependent drainage. Negative pressure bottles have been shown to average roughly -100 mmH2O of negative pressure depending on fill level, while manual aspiration is more variable.6 Both methods can evacuate fluid beyond the -20mmH2O intrapleural pressure threshold recommended based on data from multiple small studies5,6 as well as the -40mmH2O threshold, which has been shown to lead to re-expansion edema in animal studies.7
Case Outcome
The patient's respiratory status continued to decline in the MICU, and she died on day 6 of admission from respiratory failure after transition to comfort care.
Take-Home Points
- Consider all causes of hypoxemia in patients with a pleural effusion. Consider using A-a gradient to aide in diagnosis.
- Consider pneumothorax ex vacuo in your differential of pleural air after large volume thoracentesis. It is a benign process; chest tubes are not helpful.8
- Manometry may be helpful during thoracentesis to help confirm diagnosis, or to prevent excessive fluid removal that increases pleuritic pain without improving respiratory status.
- There are no hard and fast rules for therapeutic thoracentesis in the ED setting but there are some accepted best practices.
- Consider limiting volume evacuation to 1 L, as this should help any acute respiratory issues from compressive atelectasis and V/Q mismatching, limiting complications.
- Avoid negative pressure bottles and use manual aspiration or gravity drainage.
References
1. Heffner JE, Brown LK, Barbieri CA. Diagnostic value of tests that discriminate between exudative and transudative pleural effusions. Chest. 1997;111(4):970-980.
2. Pereyra MF, Ferreiro L, Valdés L. Pulmón no expansible. Archivos de Bronconeumologia. 2013;49(2):63-69.
3. Feller-Kopman D, Parker MJ, Schwartzstein RM. Assessment of pleural pressure in the evaluation of pleural effusions. Chest. 2009;135(1):201-209.
4. Maldonado F, Mullon JJ. Counterpoint: should pleural manometry be performed routinely during thoracentesis? No. Chest. 2012;141(4):846-848.
5. Galal M, et al. Pleural manometry in pleural effusion. Egyptian Journal of Chest Diseases and Tuberculosis. 2016;65(2):415-419.
6. Alraiyes AH, et al. How Much Negative Pressure Are We Generating During Thoracentesis? Ochsner J. 2017;17(2):138-140.
7. Pavlin J, Cheney FW. Unilateral pulmonary edema in rabbits after re-expansion of collapsed lung. J Appl Physiol Respir Environ Exer Physiol. 1979;46(1):31–35.
8. Heidecker J, et al. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184.