STEMI equivalents represent coronary occlusion without meeting the traditional STE criteria. It's important important to recognize these patterns in a timely fashion.
Case Introduction
A 50-year-old male with a past medical history of hyperlipidemia presented to the ED after an out of hospital cardiac arrest. 911 was activated after the patient had a syncopal episode while riding his bicycle. EMS reports that the patient was diaphoretic and reported substernal chest pain and nausea.
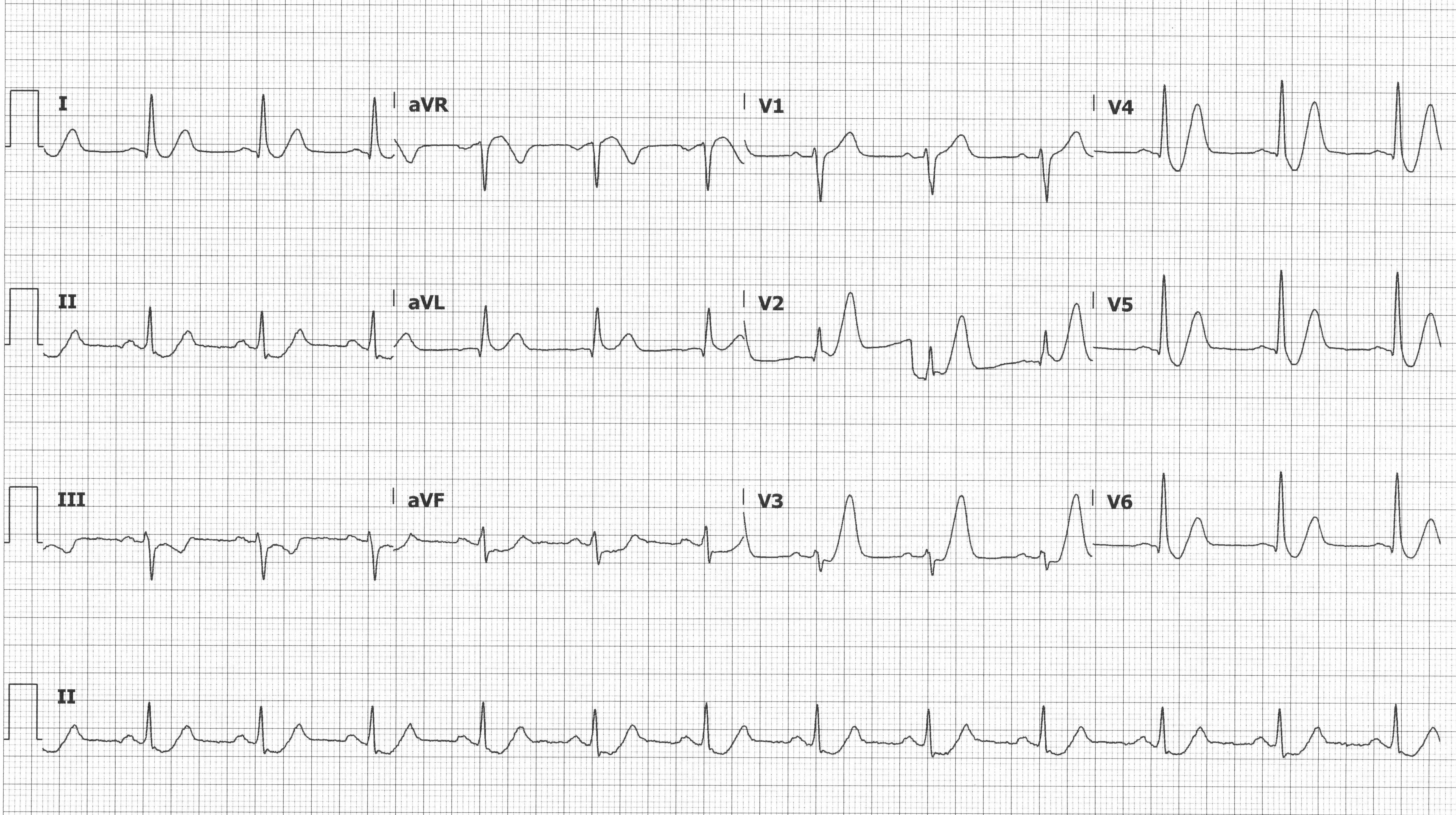
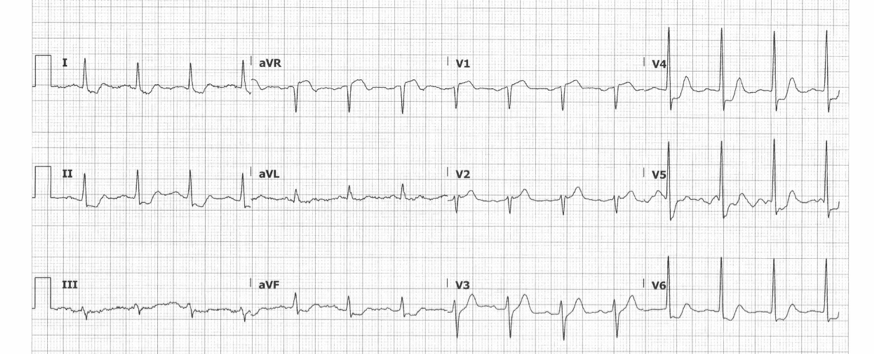
En route to the ED, the patient lost pulses, and ROSC was obtained after 1 round of CPR and a single defibrillation. Upon presentation to the ED, the patient was alert and oriented with ongoing chest pain. Initial vitals were stable. Initial ECG is shown below.
What would be your next step in management?
STEMI Criteria
Acute Coronary Syndrome is “bread and butter” emergency medicine, and emergency physicians must excel at early recognition of cardiac ischemia, as time is myocardium. Recognizing ischemic patterns on ECG is essential for identifying coronary occlusion early and facilitating emergent catheterization. STEMIs represent myocardial ischemia caused by complete occlusion of an epicardial coronary artery, often because of atherosclerotic plaque rupture and subsequent thrombosis formation. STEMI criteria have been developed to identify the patient population that benefits from emergent catheterization and revascularization.1,2 ACCF/AHA guidelines recommend first medical contact to PCI device time (commonly referred to as door to balloon time) of less than 90 minutes. If time to PCI will exceed 120 minutes, fibrinolytic therapy is recommended with 30 minutes of hospital arrival in the absence of contraindications.2
Per the 2018 AHA “Fourth Universal Definition of Myocardial Infarction,” ECG manifestations suggestive of MI (in the absence of LVH and BBB) include:
- ≥ 2.5 mm STE in V2-V3 for males < 40 years*
- ≥ 2 mm STE in V2- V3 for males ≥ 40 years*
- ≥ 1.5 mm STE in V2-V3 for females regardless of age*
- ≥ 1 mm STE all other leads
*New J-point elevation ≥ 1 mm from prior ECG should be considered ischemic
The J-point is defined as the junction between the QRS termination and the ST-segment onset, and the ST-segment should be measured against the isoelectric TP segment (assuming a stable baseline).1
STEMI Equivalents
STEMI equivalents represent coronary occlusion without meeting the traditional STE criteria and are equally important to recognize in a timely fashion. Emergency physicians must know to involve interventional cardiologists for patients with dynamic ECG changes, persistent ischemic chest pain, hemodynamic instability, and STEMI equivalent patterns that require emergent PCI to minimize morbidity and mortality.
de Winter T-waves
This ECG pattern was described in 2008 by de Winter RJ, et al. in 2008 with a case series that described this pattern occurring in approximately 2% of LAD occlusions.3 This pattern, as seen on ECG #1, demonstrates > 1 mm of upsloping STD and tall symmetric T-waves, most commonly in the precordial leads. There may be 0.5-1 mm STE in aVR, but there should be no STE in the precordial leads. Patients with this ECG pattern and a presentation concerning for ACS warrant immediate revascularization.4
Suggested occlusion: LAD
Wellen's Syndrome
In 1979 Gerson MC, et al. described exercise-induced U-wave inversions during exercise that were highly predictive of proximal LAD disease.5 The term Wellen’s syndrome is used describe these ECG changes, which represent a reperfusion pattern in the setting of severe proximal LAD stenosis, when seen in an asymptomatic patient who was recently symptomatic. There are 2 patterns seen with Wellen’s syndrome: Type A has biphasic T-waves, and Type B has deep, symmetric T-wave inversions. Diagnosis requires preserved R-wave progression, no precordial Q-waves, minimal STE, and the characteristic T-waves in the precordial leads (typically V2-V3). Treatment is coronary catheterization; stress testing is contraindicated because of the high risk of precipitating infarction.6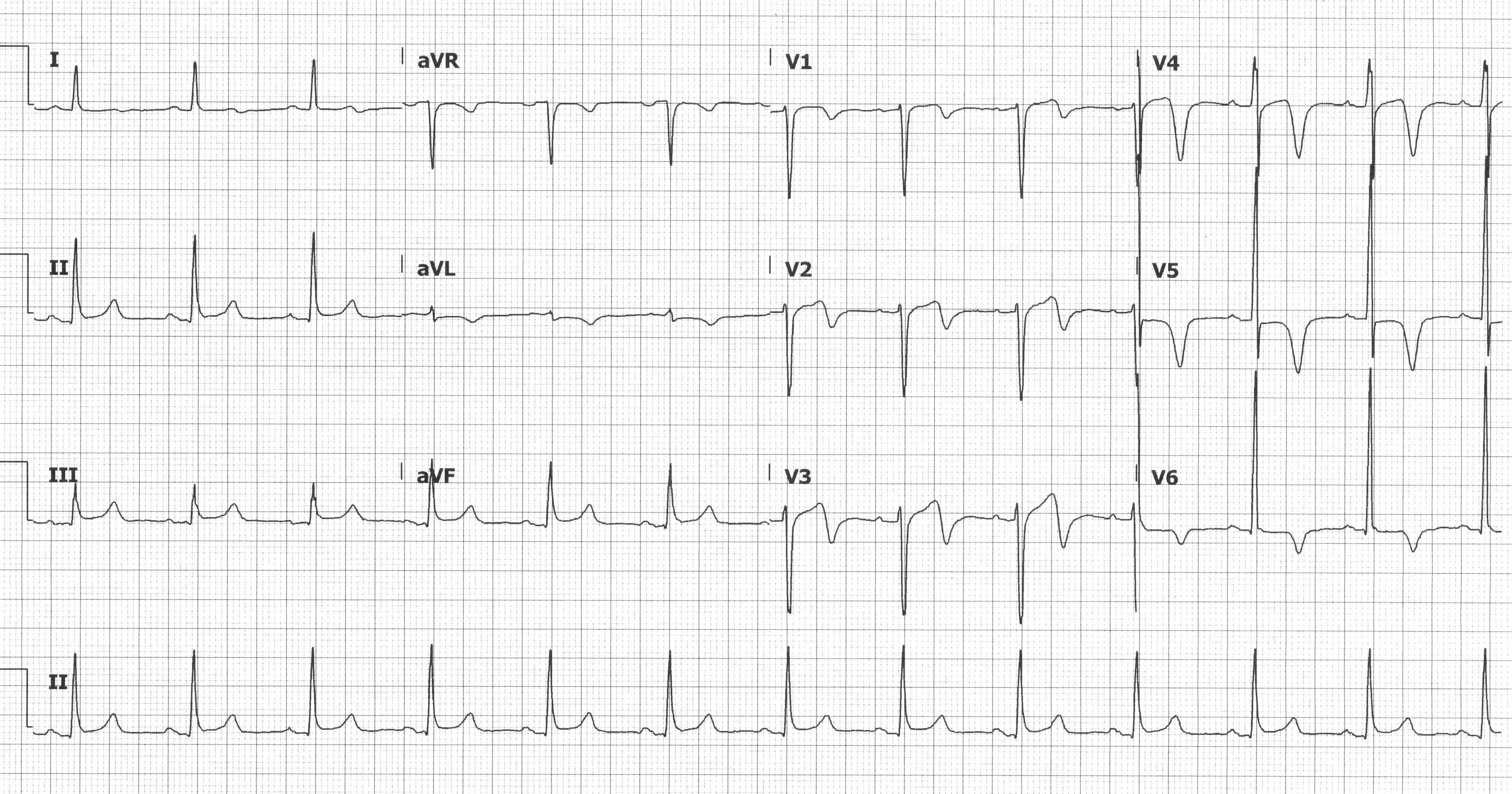
Biphasic T waves in anterior precordial leads suggesting Wellen’s Syndrome
Posterior MI
Posterior MIs are easily missed because of the absence of any STE. Posterior involvement is estimated to occur in 15-21% of all acute myocardial infarctions8 and in isolation ~3% of the time, typically due to occlusion of the left circumflex or right coronary arteries. Since there are no leads that look directly at the posterior wall, the associated ECG changes are reciprocal changes seen in the anterior leads V1-V3.7 Typical ECG changes include STD (reciprocal STE), tall R-waves (reciprocal Q-waves), and prominent positive T-waves (reciprocal terminal T-wave inversions). A posterior ECG should be obtained, and STE ≥ 0.5 mm (≥ 1 mm in men < 40 years) in V7, V8, or V9 is diagnostic of a posterior MI. Note that the absence of elevations in the posterior leads does not exclude a posterior MI.
Suggested occlusion: LCx or RCA
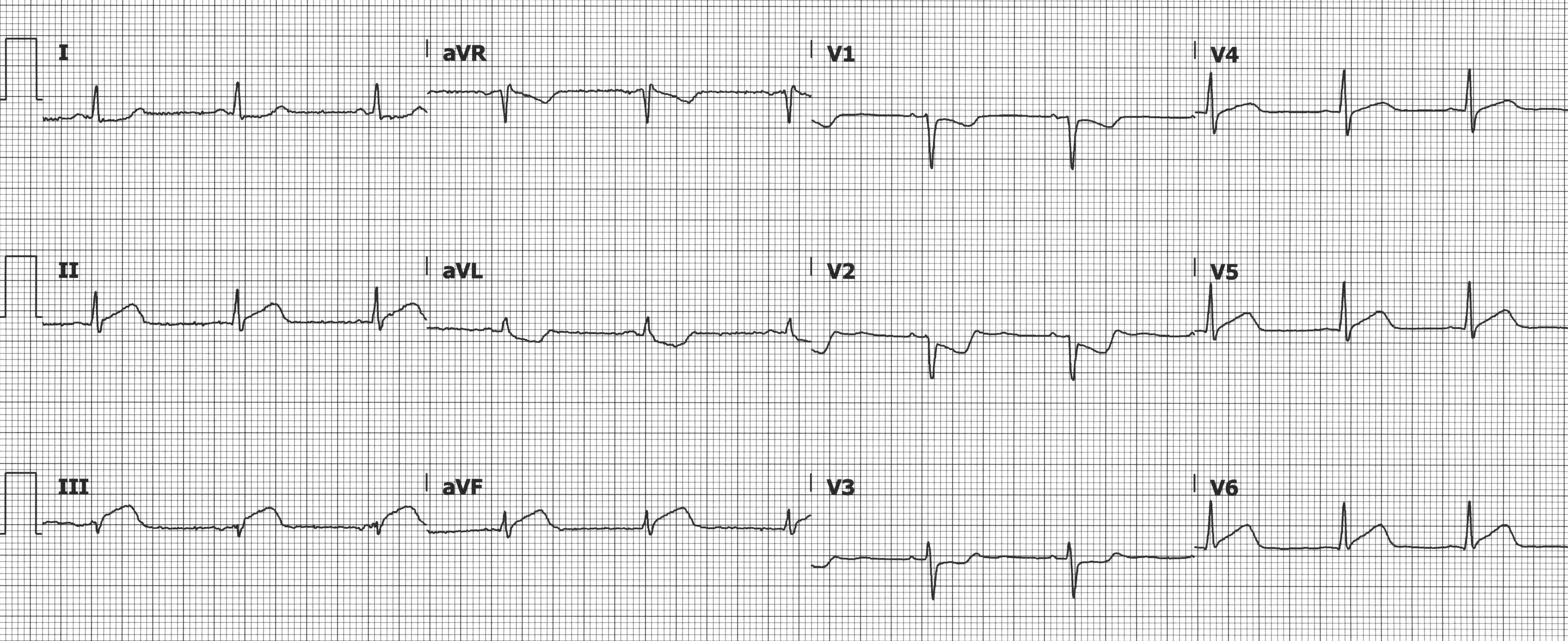 Inferior MI with STD in V1-V3 suggesting concurrent posterior MI
Inferior MI with STD in V1-V3 suggesting concurrent posterior MI
Right Ventricular MI
While isolated RV MIs are uncommon, ~1/3 of inferior MIs have RV involvement.7 The importance of recognizing RV involvement is that these patients are preload dependent. Treatment is often IV fluids and inotropes. Medications that decrease preload (eg, nitroglycerin, beta blockers, morphine) can precipitate hypotension and should be avoided or used judiciously. RV MI should be suspected when there is concurrent STE in V1 (which can be masked by a concurrent posterior MI) but should be considered in any inferior MI. A right-sided ECG should be obtained, and STE ≥ 0.5 mm (≥ 1 mm in men < 30 years) in V3R or V4R is diagnostic of a RV infarction.1 Note that the absence of elevations in the right-sided leads does not exclude a RV MI.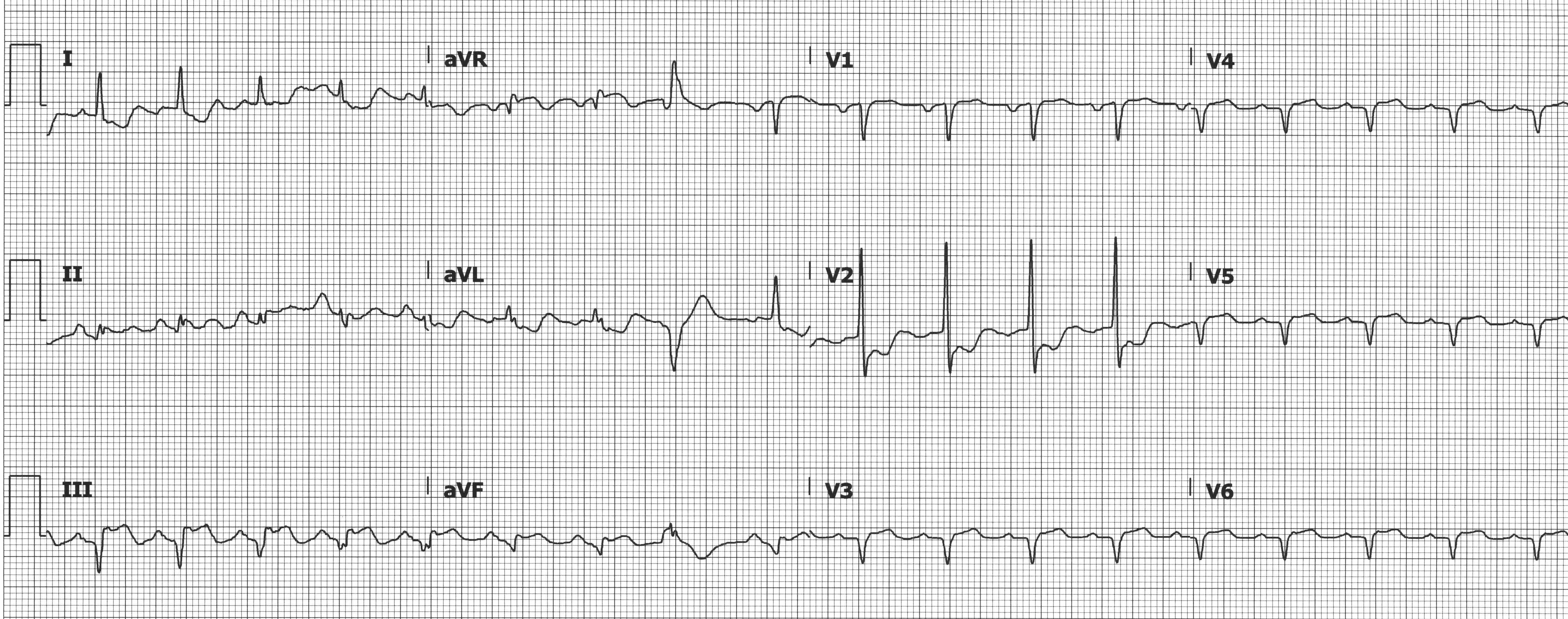
Inferior MI with STE in the right-sided leads (V3-V6 = V3R-V6R) indicating RV involvement
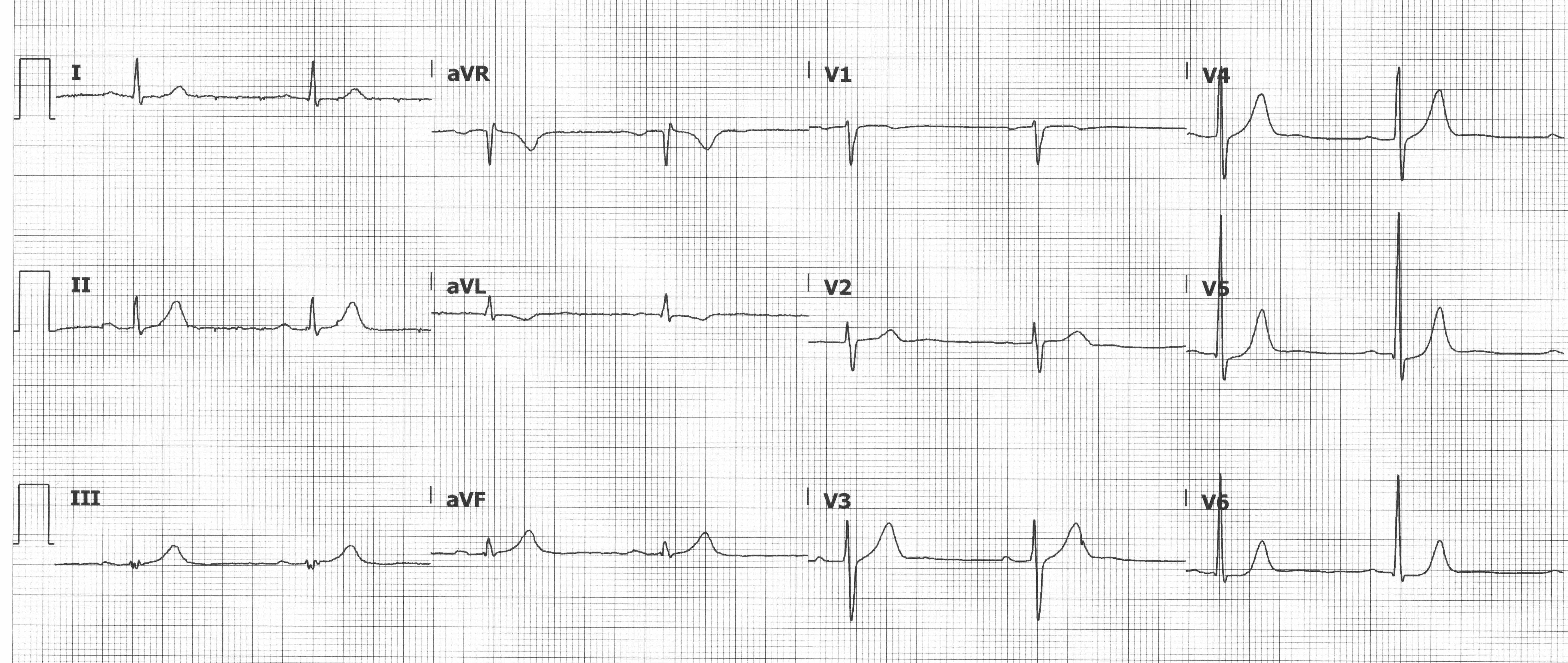
STE aVR with Diffuse STD
STE ≥ 1 mm in aVR or V1 with STD ≥ 1 mm in ≥ 6 leads can suggest left main coronary artery insufficiency, proximal LAD insufficiency, or triple vessel disease, especially if accompanied by pathologic Q-waves, hemodynamic compromise, and/or refractory symptoms. STD are most prominent in the inferior and lateral leads and thought to represent subendocardial ischemia. This ECG pattern is not specific to LMCA/proximal LAD insufficiency and can be seen in other conditions (eg, pulmonary embolism, aortic dissection, LVH with strain pattern). The 2013 ACCF/AHA Guidelines for the Management of STEMI recommend invasive angiography for patients with this ECG pattern and a presentation concerning for ACS.
Suggested insufficiency: LMCA, proximal LAD, triple vessel
 STE in aVR and V1 with diffuse STD suggesting LMCA/proximal LAD insufficiency or triple vessel disease
STE in aVR and V1 with diffuse STD suggesting LMCA/proximal LAD insufficiency or triple vessel disease
TWI in aVL
The isolated TWI in aVL is associated with both impending inferior wall MI and mid-LAD lesions.
Birnbaum Y et al. described reciprocal changes in aVL occurring in most patients with inferior wall MIs, and in some cases it was the only early indicator of an impending MI.13 Hassen et al. describes TWI in aVL as also predictive of a mid-LAD lesion.14 It is important to look for reciprocal changes when evaluating ECGs for signs of ischemia and to have a low threshold for obtaining serial ECGs, especially if there are non-diagnostic but concerning findings.
Suggested occlusion: RCA, LCx, LAD
TWI in aVL and hyperacute T-waves in the inferior leads seen in an early inferior MI
Hyperacute T-waves
Hyperacute T-waves in ≥ 2 contiguous leads may be the first signs of a developing infarct, often preceding any STE.1 Hyperacute T-waves appear broad based, often asymmetric with a more gradual upstroke than downstroke, and tall relative to the associated QRS complex. An upright T-wave in V1 > V6, described as loss of precordial T-wave balance, is considered hyperacute and concerning for ischemia especially if this is a new finding.15 A new upright T-wave in lead V1 in the absence of a LBBB or LVH is abnormal and may represent ischemia. Manno et al. described an upright T-wave in V1 as often associated with left circumflex disease.16 These findings warrant close observation, serial ECGs, and ruling out hyperkalemia as the cause of the ECG changes.
Suggested occlusion: Left circumflex
Case Conclusion
The patient’s ECG was notable for diffuse STD and tall prominent T-waves in the anterior precordial leads, concerning for De Winter T-waves. The patient was placed on telemetry, defibrillator pads applied, IV access obtained, and aspirin was administered. Interventional cardiology was called stat and immediately took the patient to the catheterization lab. Coronary angiography revealed a 100% occlusion of the proximal LAD, which was successfully stented (see figures 1 and 2). The patient was discharged 2 days later on appropriate medical therapy.
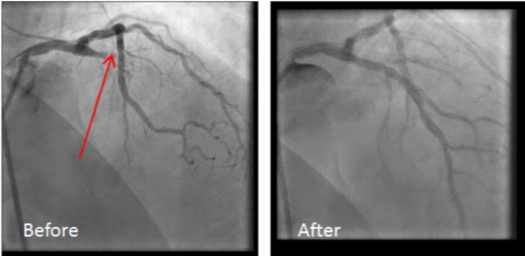
Figures 1 and 2: Pre- and post-stenting angiography
Take-Home Points
It is important to remember that an ECG is not 100% sensitive for coronary occlusion and that the absence of STE does not rule out an MI. This case is an excellent example of a patient requiring PCI even though his ECG didn’t meet STEMI criteria.
Always remember that you are treating the patient in front of you (not their ECG), serial ECGs are your friend, and the 2014 AHA/ACC Guidelines for Management of Patients with Non-ST-Elevation ACS recommend early (within 2 hours) invasive strategies for patients with hemodynamic instability, signs of heart failure, ventricular dysrhythmias, or angina refractory to medical management.
References
1. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of MI (2018). Circulation. 2018;138(20):
2. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485-510.
3. de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA. (2008). A New ECG Sign of Proximal LAD Occlusion. N Engl J Med. 2008;359(19):2071-2073.
4. Rokos IC, French WJ, Mattu A, Nichol G, Farkouh ME, Reiffel J, Stone GW. Appropriate Cardiac Cath Lab activation: Optimizing electrocardiogram interpretation and clinical decision-making for acute ST-elevation myocardial infarction. Am Heart J. 2010;160(6):995-1003.
5. Gerson MC, Phillips JF, Morris SN, McHenry PL. Exercise-induced U wave inversion as a marker of stenosis of the left anterior descending coronary artery. Circulation. 1979;60(5):1014-1020.
6. Rhinehardt J, Brady WJ, Perron AD, Mattu A. Electrocardiographic manifestations of Wellens syndrome. Am J Emerg Med. 2002;20(7):638-643.
7. Dubin D. Rapid Interpretation of EKGs. 6th ed. Tampa: Cover Publishing Company.
8. Van Gorselen E, Verheugt F, Meursing B, Oude Ophuis A. Posterior myocardial infarction: The dark side of the moon. Neth Heart J. 2007;15(1):16-21.
9. Levis JT. ECG Diagnosis: Isolated Posterior Wall Myocardial Infarction. Perm J. 2015;19(4):e143-e144.
10. Nagam MR, Vinson DR, Levis JT. ECG Diagnosis: Right Ventricular Myocardial Infarction. Perm J. 2017;21:16-105.
11. Williamson K, Mattu A, Plautz CU, Binder A, Brady WJ. Electrocardiographic applications of lead aVR. Am J Emerg Med. 2006;24(7):864-874.
12. Schwaiger JP, Mair J. Left main occlusion – a classic electrocardiogram. Wien Klin Wochenschr. 2016;128:521-523.
13. Birnbaum Y, Sclarovsky S, Mager A, Strasberg B, Rechavia E. ST segment depression in aVL: A sensitive marker for acute inferior myocardial infarction. Eur Heart J. 1993;14(1):4-7.
14. Hassen GW, Costea A, Smith T, et al. The Neglected Lead on Electrocardiogram: T Wave Inversion in Lead aVL, Nonspecific Finding or a Sign for Left Anterior Descending Artery Lesion? J Emerg Med. 2014;46(2):165-170.
15. Tewelde SZ, Mattu A, Brady WJ. Pitfalls in Electrocardiographic Diagnosis of Acute Coronary Syndrome in Low-Risk Chest Pain. West J Emerg Med. 2017;18(4):601-606.
16. Manno BV, Hakki A, Iskandrian AS, Hare T. Significance of the upright T wave in precordial lead V1in adults with coronary artery disease. J Am Coll Cardiol. 1983;1(5):1213-1215.
17. Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle branch block. N Engl J Medd. 1996;334(8):481-487.



