Sepsis is life-threatening, multi-system organ failure caused by dysregulation of immune response to infection. It causes mortality in as many as 25% of cases.1
Sepsis is common. As many as 75% of sepsis cases are seen in the emergency department.2 Care pathways and algorithms utilizing different systemic inflammatory response syndrome (SIRS) criteria to establish sepsis alerts in EDs have been widely implemented in hopes of improving patient care.2 These care pathways are important because, in some cases, they save lives.
The main pathways for sepsis identification are automated triage criteria, care teams, and sepsis pathways. Sepsis pathways are the most integrative but ultimately rely on clinical assessment and reassessment.3
Sepsis identification tools have many criticisms and vulnerabilities. Common criticisms include: lack of sensitivity and/or specificity; differing criteria commonly used in different departments and over time; utility of sepsis care pathways that ultimately rely on clinical assessment among specialty-trained emergency physicians; and vulnerabilities of overtreatment and alert fatigue.3
A systematic review showed consistently high negative predictive value (99-100%); meanwhile, it found highly variable sensitivity (10-100%), specificity (78-99%), positive predictive value (5.8-54%), and primary outcomes (time to antibiotics, length of stay, and improved mortality).1
Another study showed that computer alerts identifying sepsis patients with >2 SIRS criteria in the ED increased lactate testing, but had no change in inpatient mortality.5
The value of different sepsis care pathways has been a major area of research and discussion among clinicians. This has caused large variability in care pathway utilization and differing criteria: SIRS, CMS, Surviving Sepsis Campaign, qSOFA, SOFA, Sepsis 3, Shapiro criteria, CEC SEPSIS KILLS, and differing hospital-based pathways, to name a few.6,7
Currently, the most widely accepted criteria is Sepsis 3, which establishes SIRS as the most sensitive sepsis criteria and qSOFA as more specific to sepsis.6,8,9,10 Sepsis 3 also eliminated the prior “severe sepsis” classification as redundant nomenclature. Sepsis 3 defines septic shock as end-organ damage based on refractory hypotension (>65mmHg MAP without vasopressors) or initial elevated lactate (>2.0 mmol/L (18 mg/dL).
Septic shock is an important distinction from sepsis as it is associated with a significant increase in mortality up to 40%.10 Sepsis 3 also recommends use of a SOFA score (different than qSOFA) to calculate mortality risk once sepsis is diagnosed.8,10
Based on the variation of criteria, likely all are suboptimal for use in the emergency setting, and most include phrases that rely on clinician assessment, such as: “Clinicians should not be restricted to definition criteria when evaluating patients with infection and should wisely use the broad array of information obtained by rigorous clinical observation.”7,10
Case Report
Our patient was a previously healthy 24-year-old male brought in by ambulance for one day of shortness of breath, productive cough, and fever. A sepsis alert was activated by EMS.
The patient reportedly had an upper respiratory infection (URI) two weeks ago. A SARS-Cov-2 test was negative. He reported feeling better after a week, and then feeling sick again the night prior. He tried two puffs of his albuterol inhaler, given for his URI, took Nyquil, and went to bed.
He called 911 in the morning for increasing shortness of breath and cough. EMS found the patient to have an oxygen saturation of 80% on room air, and 92% on 4 liters of oxygen by nasal cannula. The patient said he had chest discomfort, which he described as shortness of breath rather than pain. He denied chills, rhinorrhea, sore throat, leg swelling, abdominal pain, dysuria, joint swelling, myalgias, or rash.
The patient reported having left foot surgery six weeks prior for an ankle fracture. Upon examination, no acute tenderness to the left foot was found. There was no history of pneumonia, and the patient denied personal and family history of clotting. He had received two SARS-Cov-2 vaccines. A review of systems was positive for pallor, but otherwise negative.
On presentation, the patient’s vital signs were: blood pressure 97/63 mmHg; pulse 138; temperature 37.7 C (99.8 F); respiratory rate 28; oxygen saturation 92% on 4 liters oxygen via nasal cannula; height 1.778m (5’10”); weight 83.9 (185 lbs); and BMI 26.54 kg/m^2.
On physical exam, the patient was in acute respiratory distress and toxic appearing. He was tachycardic and tachypneic; normal rhythm, S1 and S2 heard, no friction rub, murmurs, or gallops. He had chest wall tenderness and bilateral rhonchi; no wheezes or stridor. He had a flat, nontender abdomen, with normal bowel sounds. His left foot was mildly tender to dorsiflexion (recent left foot surgery). There was no erythema, bruising, warmth, or color change; range of motion was intact. There was upper left thigh tenderness to palpation; no redness or swelling. Skin was pale and dry with 3-second capillary refill. The patient was alert and oriented, with no focal neurologic deficit.
This patient met SIRS criteria. Sepsis order set — including blood cultures, lactate, and IV Rocephin — was ordered. EKG, troponin, CXR, and UA: EKG returned with sinus tachycardia; WBC 14.4 with left shift. A complete metabolic panel was within normal limits and SARS-Cov-2 negative. Troponin was elevated to 0.07, indicating heart strain. Lactate was within normal limits at 1.66. CXR returned without acute process. A CT angiogram was ordered for pulmonary embolus evaluation. Wells' criteria placed him in the moderate risk group.
During CTA, the patient required increasing oxygen to 8L with 87% oxygen saturation. BP was 126/64. The CTA showed bilateral massive PE with left lower lobe consolidation. Pulmonology and IR were consulted.
Oxygen requirement increased to 60L/min at 80% FiO2 by Airvo. Pulmonology recommended heparin and procedure for thrombectomy versus catheter-directed thrombolysis. The patient was accepted to the ICU. He was notified and re-questioned for clot risk. The patient reported groin pain starting two weeks ago at the time of his URI; surgery to left foot was six weeks ago.
The patient subsequently underwent thrombectomy with inferior vena cava filter placement and lower extremity ultrasound that revealed a left femoral deep vein thrombosis. He was admitted to the ICU and placed on heparin to dissolve the femoral clot.
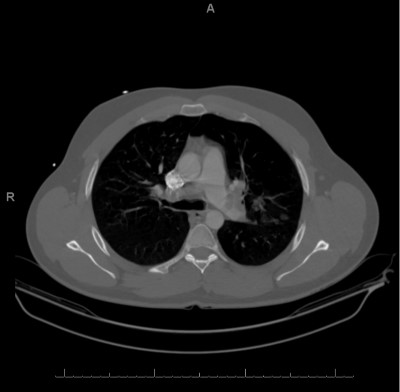
Computed tomography angiography. IMPRESSION: (1) Extensive bilateral pulmonary emboli involving the central and peripheral pulmonary tear system. There is mild straightening of the interventricular septum, which may represent increased right heart pressures. No dilatation of the pulmonary trunk is identified. (2) Focal consolidations in the bilateral upper lungs and left lower lung. This may represent a multifocal infectious process versus sequelae of pulmonary infarcts.
Discussion
This case report emphasizes clinical decision pathways and potential outcomes of the emergency physician’s clinical reasoning.
This patient met SIRS criteria for tachycardia, hypotension, fever, and WBC 14.4 with left shift. He initially met sepsis criteria with a suspected source of pneumonia based on recent history of upper respiratory infection, physical exam finding of rhonchi, and non-specific consolidation seen on chest X-ray.
He no longer met criteria after sepsis laboratory testing demonstrated no organ dysfunction.
Based on his increasing oxygen requirement, a CT angiogram was ordered to evaluate for pulmonary embolism, which was ultimately diagnosed. This was a previously healthy 24-year-old male whose only risk factor for pulmonary embolus was ankle surgery six weeks prior to presentation.
Did common sepsis care pathways improve or hinder his care?
Are there different types of care pathways that could have been better applied to this patient?
What role should clinical care pathways play in diagnosis of life-threatening conditions?
Sepsis alert criteria is a well-validated, performance-based measure. This allows for prompt treatment with antibiotics, which has been shown to improve morbidity and mortality of sepsis.3 It’s important to note that performance-based measures depend on arriving at the final diagnosis of sepsis. If a patient presents with a chief complaint non-specific to sepsis and is ultimately found not to be septic, these alerts do not inform treatment.
As discussed above, criteria to enter sepsis care pathways are highly sensitive, but non-specific. This methodology neglects the quintessential job of an emergency physician — to triage undifferentiated complaints for diagnosis and treatment decisions with limited information. Diagnosis-based performance measures don’t capture the risk stratification of this task, concomitant evaluation of multiple life-threatening diagnoses, or patients for whom a diagnosis was considered but safely ruled out.3
Chief-complaint based measures are a potential solution when applied to the correct patient population.3 One such example is the use of EKG and counseling on smoking cessation in the evaluation of chest pain in the ED. This represents a diagnostic and population-based intervention applied to a chief complaint.3 Our patient may have benefited from a chief-complaint based performance measure, as he had shortness of breath with mixed risk factors for separate life-threatening diagnoses: sepsis and PE.
Some limitations of chief-complaint based measures are the discrepancy in labeling of the chief complaint.3 This may include DSM diagnoses vs. common language chief complaints, provider discrepancy between similar chief complaints (shortness of breath vs. dyspnea), or separate but related chief complaints.3
Another criticism discussed in the literature is the utility of care pathways among emergency physicians. Historically, non-emergency physicians — trained in another specialty such as family medicine or internal medicine — staffed the ED. Concomitantly, sepsis was often a missed or delayed diagnosis. Interprofessional differences in perception of causes for delayed diagnosis led to the suggestion of written protocols.15 Sepsis recognition has since evolved via robust education campaigns, research, quality improvement measures, increased board certification of emergency providers, ED personnel training, and integration of sepsis alerts/care pathways into electronic medical records.2 Some suggest emergency physicians are likely much better at detecting sepsis, and in the highly monitored ED setting, the benefit of care pathways over clinician gestalt may diminish.2
Limitations
Many studies have shown mortality benefit from early recognition and treatment of sepsis. There is extensive conflicting research on whether sepsis alerts in the emergency department setting confer decreased mortality. There are many process-of-care measures conferring improvement with implementation of sepsis care pathways; however, high-quality studies have been unable to reliably show a mortality benefit when implemented across all patient care.2Sepsis alerts demonstrate utility, but further research is needed to identify their utility in emergency departments and build a more ideal alert system.
Conclusion
Care pathways were originally implemented to facilitate prompt diagnosis of sepsis in order to implement life-saving treatment. Since their original construction, there have been numerous conflicting methods of implementation and criteria based on debate of sensitivity, specificity, and utilization in different emergency departments. There is also some evidence that well-trained ED personnel need not rely on care pathways for recognition of sepsis to improve mortality. More critically, care pathways may neglect the core competencies of the board-certified emergency physician to recognize life-threatening diagnoses while maintaining a broad differential diagnosis and implement treatment based on limited information.
Discussion of chief-complaint based protocols are a potential solution to better optimize sepsis care pathways in the ED. Further research is needed to establish the most optimal utilization of sepsis care pathways. Extension of this discussion to other care pathways is recommended to further patient care in an emergency setting.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304-377.
- Hwang MI, Bond WF, Powell ES. Sepsis Alerts in Emergency Departments: A Systematic Review of Accuracy and Quality Measure Impact. West J Emerg Med. 2020;21(5):1201-1210.
- Griffey RT, Pines JM, Farley HL, et al. Chief complaint-based performance measures: a new focus for acute care quality measurement. Ann Emerg Med. 2015;65(4):387-395.
- Uffen JW, Oosterheert JJ, Schweitzer VA, Thursky K, Kaasjager HAH, Ekkelenkamp MB. Interventions for rapid recognition and treatment of sepsis in the emergency department: a narrative review. Clin Microbiol Infect. 2021;27(2):192-203.
- Berger T, Birnbaum A, Bijur P, Kuperman G, Gennis P. A Computerized Alert Screening for Severe Sepsis in Emergency Department Patients Increases Lactate Testing but does not Improve Inpatient Mortality. Appl Clin Inform. 2010;1(4):394-407.
- Olson Z, Estesphan M. Clinical Curriculum. 2022. Accessed 13 May 2022.
- Sparks R, Harada A, Chavada R, Trethewy C. Comparison of different sepsis scoring systems and pathways: qSOFA, SIRS, Shapiro criteria and CEC SEPSIS KILLS pathway in bacteraemic and non-bacteraemic patients presenting to the emergency department. BMC Infect Dis. 2022;22(1):76.
- Mdcalc.com. 2022. MDCalc - Medical calculators, equations, scores, and guidelines. Accessed 13 May 2022.
- Donaldson, R. 2016. Portal:Categories - WikEM. Accessed 13 May 2022.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
- Salomão R, Ferreira BL, Salomão MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: evolving concepts and challenges. Braz J Med Biol Res. 2019;52(4):e8595.
- Guirgis FW, Jones L, Esma R, et al. Managing sepsis: Electronic recognition, rapid response teams, and standardized care save lives. J Crit Care. 2017 Aug;40:296-302.
- Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011 Mar;39(3):469-73.
- Gatewood MO, Wemple M, Greco S, Kritek PA, Durvasula R. A quality improvement project to improve early sepsis care in the emergency department. BMJ quality & safety. 24(12):787-795.
- Burney M, Underwood J, McEvoy S, et al. Early detection and treatment of severe sepsis in the emergency department: identifying barriers to implementation of a protocol-based approach. [Published correction appears in J Emerg Nurs. 2013 Jan;39(1):106]. J Emerg Nurs. 2012;38(6):512-517.