Spontaneous coronary artery dissection and Takotsubo syndrome rarely occur in the same patient at the same time - but when that patient appears, will you be able to act decisively?
A healthy, 35-year-old female with a past medical history of anxiety and rheumatoid arthritis presented to the emergency department for 8/10 substernal chest pressure and dizziness that began 45 minutes prior to arrival. Her chest discomfort began while arguing with her brother at a family gathering. She initially attributed her symptoms to anxiety. She developed more severe chest discomfort and dizziness while preparing to leave the party and yelling at her son to put on his shoes. She denies nausea, diaphoresis, or dyspnea. There was no pain radiation or tearing quality. The patient has no history of diabetes, hypertension, tobacco abuse, or family history of premature coronary artery disease. She denies alcohol or illicit drug use.
On initial evaluation, the patient was mildly hypertensive (BP 141/93), but otherwise hemodynamically stable. The initial ECG showed ST-elevation in leads II, III, aVF, V5, and V6 (Figure 1), indicating inferolateral wall injury pattern. An immediate point-of-care echocardiogram was performed that demonstrated normal chamber size and contractility. There was no pericardial effusion or obvious wall motion abnormality, including apical ballooning, which was highly suspected based on the patient’s age and history of present illness. A cardiologist was consulted, and the patient was taken immediately to the cardiac catheterization laboratory. Her initial troponin-T was 0.037 ng/mL (just 0.007 above the upper limit of normal). Other routine labs, including a creatinine, were unremarkable. Left ventriculography revealed an ejection fraction of 30-35% with left ventricular apical ballooning, consistent with Takotsubo syndrome.
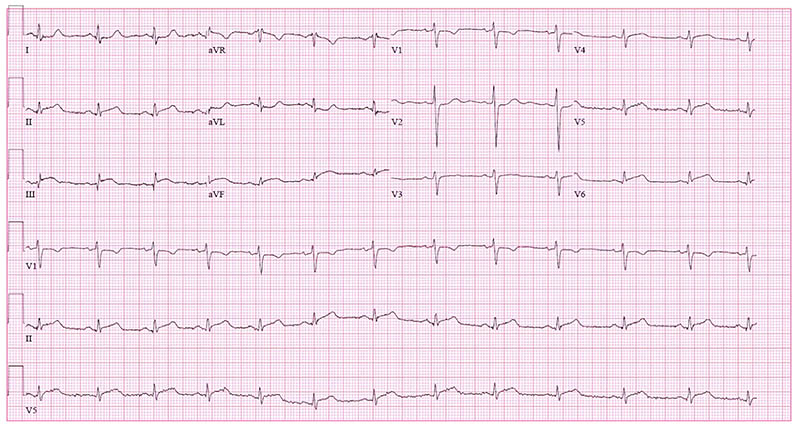
Figure 1. Initial ECG
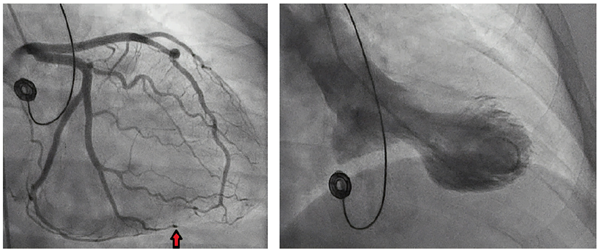
Coronary angiography revealed an abrupt occlusion of the distal branch of the obtuse marginal branch of the left circumflex. A linear lucency was noted, suggesting a dissection and hematoma.
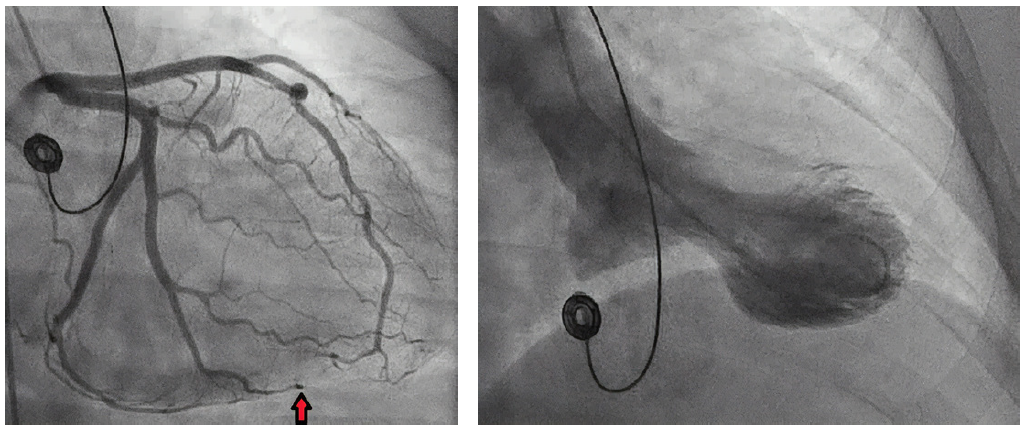
Figure 2. Still image from left coronary angiogram showing spontaneous dissection. (left)
Figure 3. Still image from left ventriculogram demonstrating apical ballooning. (right)
Diagnosis: Simultaneous spontaneous coronary artery dissection (SCAD) and Takotsubo syndrome!
To date, there have only been 15 published case reports of these two diagnoses occurring simultaneously.
Case Conclusion
Following her cardiac catheterization, the patient was admitted for further workup including cardiac MRI which revealed transmural infarction of a portion of the inferolateral wall. A comprehensive echocardiogram 30 hours after arrival showed resolved wall motion abnormalities and an improved ejection fraction of 55%. Her ECG changes resolved. She was then discharged with cardiac rehabilitation.
Discussion
This case demonstrates two important causes of ST-elevation in women without atherosclerosis: spontaneous coronary artery dissection (SCAD) and Takotsubo syndrome. While Takotsubo syndrome causes ACS and ST elevation, infarction is often not found. It is estimated that Takotsubo syndrome is present in 5-10% of women with acute coronary syndrome. SCAD most often causes both ST-elevation and myocardial infarction (STEMI). A recent study showed that 24% of acute MI’s in women < 50 years old were due to SCAD. The most recent evidence also shows that both conditions are under recognized. Both conditions disproportionately affect women and are linked to acute emotional stress.
Pearl: Look for risk factors for SCAD and Takotsubo in women presenting with chest pain without a history of atherosclerosis.
Myocardial infarction in young, healthy women is rare. It would have been reasonable to attribute this patient’s chest pain to acute anxiety. This patient’s history, however, included risk factors for both SCAD and Takotsubo (Tables 1 & 2). This highlights the importance of maintaining a broad differential diagnosis and avoiding anchoring bias in the evaluation of healthy, female patients that present with chest pain.
Table 1. Risk Factors for SCAD
| Young, healthy female (age 35-55) |
| Prior vascular dissection |
| Fibromuscular dysplasia |
| Connective tissue disorders |
| Hormone therapy |
| Multiparity |
| Systemic inflammatory disease |
Table 2. Risk Factors for Takotsubo Syndrome
| Post-menopausal female |
| Recent physical or emotional stressor |
| Alcohol abuse |
| Cardiovascular disease risk factors |
| Psychiatric disease, especially anxiety |
Pearl: Use POCUS to look for apical ballooning or wall motion abnormalities.
The use of point-of-care ultrasound (POCUS) can be used to supplement the workup and evaluation among ED patients. Bedside echo can be used to look for pericardial effusion, apical ballooning, RV strain, or wall motion abnormalities. Apical and mid-ventricular ballooning and basal hypercontractility of Takotsubo syndrome can be seen best in the apical 4-chamber view. SCAD can cause more subtle changes including ventricular dyskinesia.
The patient arrived shortly after developing symptoms, and apical ballooning had not yet developed. In patients suspected for apical ballooning, consider performing a repeat echo while trending troponins for early presenters. The onset of takotsubo-induced myocardial dysfunction following an emotional stressor is unknown.
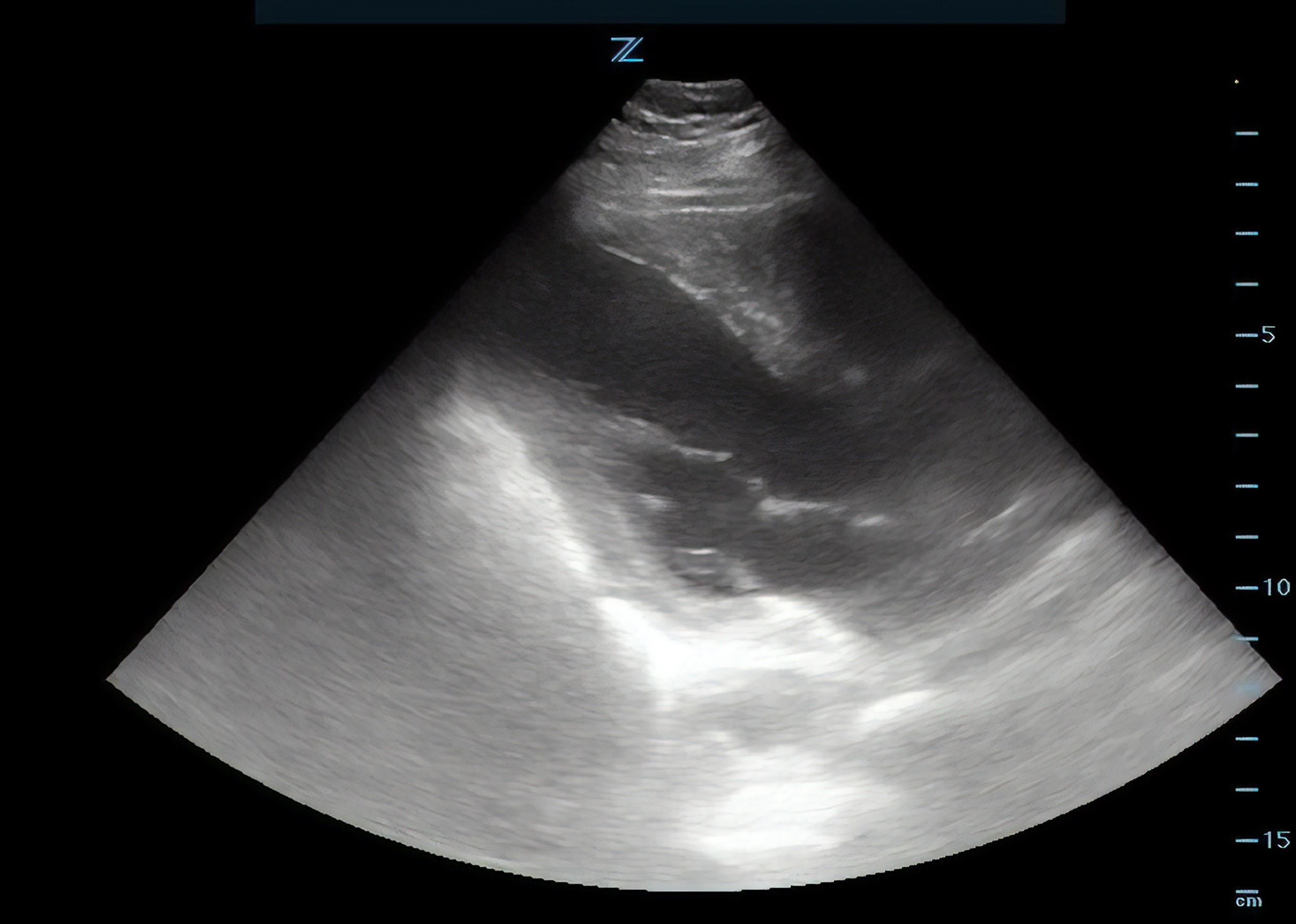
Figure 4. Still image from parasternal long axis point of care echocardiogram.
Pearl: Don’t delay ECG and troponin.
Have a low threshold to obtain an ECG in young patients presenting with chest pain and anxiety. Takotsubo syndrome alone can cause ST-elevation, particularly in the anterior precordial leads. Look closely for T wave inversion in lead aVR without corresponding T wave inversion in lead V1. SCAD may also result in ST segment elevation in the affected vessel distribution on EKG.
Both conditions also result in an elevated serum troponin.
Bottom Line
Both SCAD and Takotsubo can result in acute ST-elevation in women without atherosclerosis. A prompt ECG, troponin, bedside ECHO, and a careful medical history will help clinch the diagnosis and avoid premature anchoring bias. A cardiac catheterization is required to confirm the diagnosis.
References
1. Buccheri D, Zambelli G. The link between spontaneous coronary artery dissection and takotsubo cardiomyopathy: Analysis of the published cases. J Thorac Dis. 2017;9(12):5489-5492.
2. Cecchetto A, Dalla Chiara E, Ciccio C, et al. Spontaneous Coronary Artery Dissection and Takotsubo Cardiomyopathy: MRI Demonstration of the Underlying Mechanism. ARC Journal of Cardiology. 2018;4(2):13-14.
3. Hayes S, Kim C, Saw J, Adlam D, et al. Spontaneous coronary artery dissection: Current state of the science: A scientific statement from the American Heart Association. Circulation. 2018;137(19):e523-557.
4. Sachdev E, Merz C, Mehta PK. Takotsubo cardiomyopathy. European Cardiology Review. 2015;10(1):25.
5. Saw J, Aymong E, Mancini GB, Sedlak T, et al. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30(7):814-9.
6. Saw J, Aymong E, Sedlak T, Buller C, Starovoytov A, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645-55.
7. Tweet MS, Hayes SN, Pitta SR, Simari RD, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579.
8. Y-Hassan S, Themudo R, Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: The chicken or the egg causality dilemma. Catheter Cardiovasc Interv. 2017; 89:1215-1218.