Preparing for and performing an emergent intubation in the emergency department (ED) requires critical thinking and precision.
Once the endotracheal tube is secured and the stress of impending respiratory failure subsides, the post-intubation period may seem trivial. However, there is a growing body of literature demonstrating the importance of post-intubation care in the ED. It is therefore imperative that emergency physicians be adept at managing the sedation and analgesic needs of intubated patients in the ED.
Post-intubation care has been shown to have a major impact on hospital mortality and length of stay. As demonstrated in the 2012 SPICE trial, deep early sedation within the first four hours of intubation was found to be an independent predictor for delayed extubation and increased hospital mortality.1 Oversedation has also been shown to increase length of ICU stay.2 Various studies have demonstrated the negative effects of prolonged deep sedation including increased incidence of hospital delirium and higher six month mortality.3
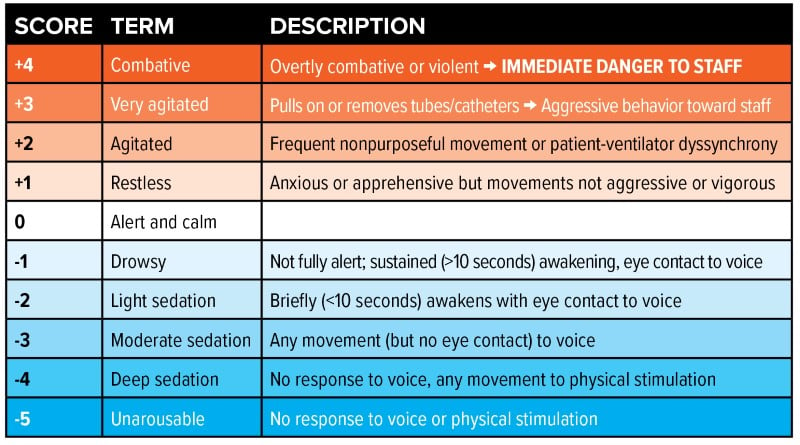
The Richmond Agitation Sedation Scale (RASS) is the most widely used tool in the ICU setting to objectively characterize the depth of sedation in the mechanically ventilated and critically ill patient (Figure 1). For patients who do not require deep sedation (eg, patients receiving neuromuscular blockade, open abdomens, status epilepticus, etc.) the goal is to titrate analgesia and sedation to a RASS between -1 to 0.4
Figure 1. Richmond Agitation Sedation Scale (RASS)
Sessler C, Gosnell M, Grap MJ, et al. The Richmond agitation-sedation scale. Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338.
In a cohort study of mechanically ventilated patients, a majority of patients (64%) were overly sedated in the ED with a median RASS of -3.0.1 The Society of Critical Care Medicine (SCCM) guidelines recommends an “analgesia-first sedation” approach when managing the sedation needs of the mechanically ventilated patients. This is the concept of optimizing pain control prior to implementation of sedation medications. The overall goal is to minimize pain while keeping the patient at a light sedation.
Analgesic treatment should be started immediately post-intubation, as patients treated with long-acting paralytics are unable to exhibit signs of discomfort. The two most commonly used neuromuscular blocking agents used during rapid sequence intubation (RSI) are rocuronium and succinylcholine. Although both produce a similar paralytic effect, their durations of action are markedly different. Succinylcholine typically lasts 4-6 minutes, and rocuronium lasts an average of 30-90 minutes depending on dosage used.6 In contrast, sedatives used in RSI have a much shorter duration of action. For example, etomidate is a commonly used sedative that has a duration of action lasting only 3-5 minutes.5 This can unfortunately create a situation where a patient remains paralyzed without receiving adequate analgesia and sedation.
This paper explores the most commonly used analgesics and sedatives, and how to pick the best post-intubation strategy for your patient.
How to Choose a Sedation Strategy
A few pearls when picking your sedation strategy:
- RASS targets may vary depending on clinical indication for sedation. Determine your post-intubation RASS goal while preparing for intubation.
- While preparing medications for intubation, discuss post-intubation analgesia and sedation with the bedside nurses/pharmacist and have analgesia and sedation ready to be started post-intubation.
- Recheck your patient’s level of sedation at 30 minutes, 60 minutes, and 90 minutes afterward to determine if any changes are necessary.
- Prepare a plan for breakthrough pain and agitation post-intubation and talk to the bedside nurse about it. Consider ordering as-needed medications after intubation to prevent delays in patient care.
- Place soft restraints on patients after intubation as a precautionary measure while titrating analgesia and sedation.
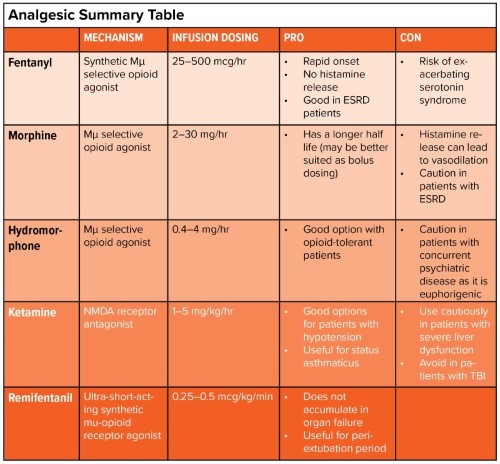
ANALGESICS
Fentanyl
Common dosages
- Bolus: 50-100 mcg q30-60min
- Infusion: 25-100 mcg/hr
Mechanism
- Synthetic Mu-selective opioid agonist (but can potentially activate delta and kappa-receptors)
- 50-100x more potent analgesia when compared to morphine
- Increased dopamine transmission
- Low affinity for postsynaptic 5-HT1A and 2A serotonin receptors
- Metabolized hepatically via the CYP450 system with 3-7 hour half-life
- Highly lipophilic
Pros
- Rapid onset, which allows for rapid dose titration
- No histamine release, therefore less hemodynamically disruptive
- No toxic metabolites that accumulate in cases of renal failure
Cons
- Constipation
- Respiratory depression
- Accumulates in fat, causing delayed awakening due to increased half-life
- Be wary of patients on CYP450 inhibitors or inducers as that can affect metabolism
CAUTIONS
- Patients with liver failure: can be used, but cautiously as metabolism is prolonged
- Patients with gastrointestinal obstruction: may compound gut motility issues
- Patients actively in serotonin syndrome: avoid completely
Morphine
Common dosages
- Bolus: 4-8 mg q1-2hr
- Infusion: 2-30 mg/hr
Mechanism
- Mu-selective opioid agonist (but can potentially activate delta and kappa-receptors)
- Glucuronidation to two metabolites in the liver
- A μ-opioid agonist
- No analgesic effect
- Morphine-6-glucuronide (M6G)
- Morphine-3-glucuronide (M3G)
Pros
- Longer half-life than other opioid agents
- Less euphoria with decreased abuse/addiction potential
Cons
- Histamine release can lead to pruritus and vasodilation (can contribute more to hypotension and bradycardia)
- Constipation
- Respiratory depression
CAUTIONS
- Patients with liver failure: can be used, but cautiously as metabolism is prolonged
- Patients with gastrointestinal obstruction: may compound gut motility issues
- Avoid in patients with renal failure
- Avoid in patients with concurrent use of monoamine oxidase inhibitors (MAOIs): can lead to hypotension, serotonin syndrome, increased respiratory depression
Hydromorphone
Common dosages
- Bolus: 0.4-1 mg q1-2hr
- Infusion: 0.4-4 mg/hr
Mechanism
- Mu-selective opioid agonist (but can potentially activate delta and kappa-receptors)
- Glucuronidation to hydromorphone-3-glucuronide in the liver (no analgesic effect)
Pros
- Longer half-life than fentanyl
- An effective option in patients tolerant of morphine or fentanyl
Cons
- Very euphorigenic and can lead to inappropriate use for anxiety or agitation
- Constipation
- Respiratory depression
CAUTIONS
- Patients with liver failure: can be used, but cautiously as metabolism is prolonged
- Patients with gastrointestinal obstruction: may compound gut motility issues
- Patients with renal failure: cautiously monitor as HM3G can accumulate and cause seizure, myoclonus, or agitation
Remifentanil
Common dosages
- Bolus: 0.5-1 mcg/kg q2-5min
- Infusion: 0.25-0.5 mcg/kg/min
Mechanism
- Ultra-short-acting synthetic mu-opioid receptor agonist
Pros
- Organ-independent metabolism (widespread extravascular metabolism by extrahepatic, nonspecific blood and tissue esterases)
- Rapid offset of action
- Allows for fast and predictable extubation
Cons
- Constipation
CAUTIONS
- Patients with gastrointestinal obstruction: may compound gut motility issues
- Not widely available for use outside of the operating room
Ketamine
Common dosages
- Bolus: 0.25 to 0.5mg/kg
- Infusion: 1-5mg/kg/hr
Mechanism
- NMDA antagonist (receptor for glutamate - the primary excitatory neurotransmitter of the brain)
- Hepatic metabolism
Pros
- Hemodynamically stable drug, although may cause mild elevation in blood pressure at higher doses
- Bronchodilator, may be helpful in status asthmaticus
- Anticonvulsant properties
- Amnestic
- Treats agitation refractory to other drugs
Cons
- Hypertension
- Bronchorrhea
- Re-emergent phenomenon
CAUTIONS
- Patients with uncontrolled hypertension
- Pregnant patients
- Severe liver dysfunction
- Active psychosis or delirium
- Avoid in patients with traumatic brain injuries - increases intracranial pressure
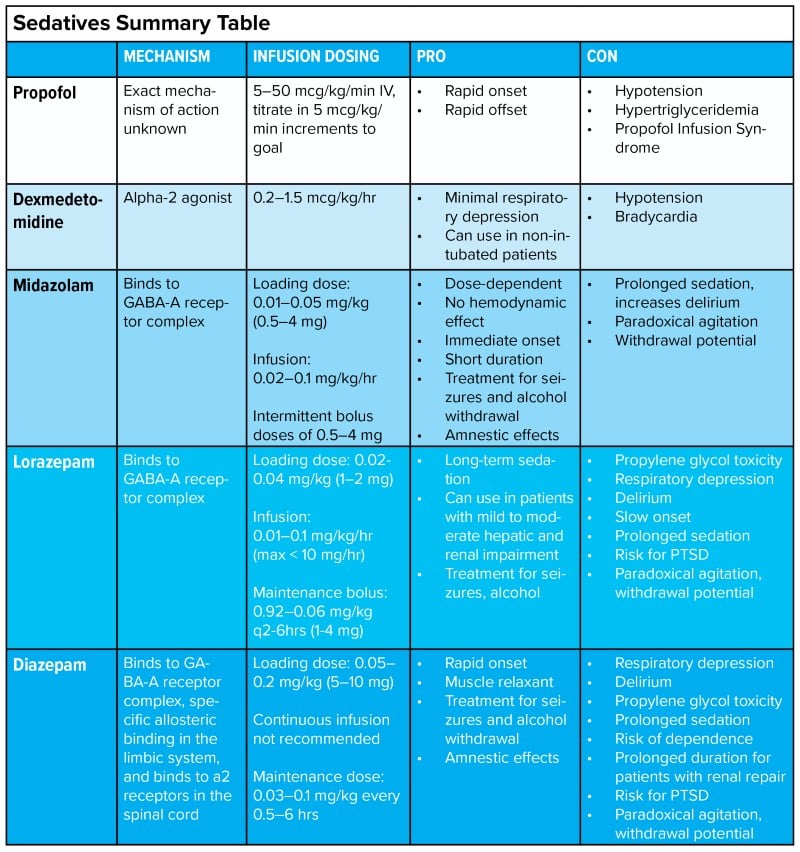
SEDATIVES
Propofol
Common dosages
- Bolus: Generally not recommended, but can consider giving 10-20 mg boluses based on hemodynamic stability
- Infusion: 5-50 mcg/kg/min IV, titrate in 5-10 mcg/kg/min increments
Mechanism
- Exact mechanism of action unknown
- Effects the GABA-mediated chloride channels in the brain. Decreases the dissociation of GABA from receptors resulting in hyper-polarization of the membrane, decreasing further successful action potentials.
Pros
- Rapid onset and offset of action - use for short procedures, patients that require frequent neurological assessment
- Shown to have a lower mortality, earlier discharge, and earlier discontinuation from mechanical ventilation compared with midazolam and lorazepam10
- Anticonvulsant properties
- Bronchodilator
- Patients do not typically develop tolerance or withdrawal
- Treats alcohol withdrawal
CAUTIONS
- Use caution with patients with depressed blood pressures, albeit can augment with vasopressor support
- Use caution with patients with pulmonary hypertension
- Avoid in patients with severe heart failure, heart block, and bradycardia
Dexmedetomidine
Common dosage
- Infusion: 0.2-1.5 mcg/kg/hr
Mechanism
- Alpha-agonist with preferential alpha-2 receptor selectivity of 1600 to 1
- Activates presynaptic alpha-2 receptors in the brainstem which provide negative feedback for neurotransmitter release
- Mechanism on peripheral nerves (peripheral nerve block) unknown but suspected to be perineural
Pros
- Provides sedation without significant respiratory depression. Can be used in patients who are not intubated (eg, NIPPV).
- Provides sedation and anxiolysis that improves patient comfort and allows patient cooperation (eg, agitated patients, patients being weaned from mechanical ventilation
Cons
- May precipitate and exacerbate hypotension and bradycardia
- Prolonged use may cause tolerance and precipitate withdrawal if suddenly discontinued. May transition to oral clonidine.
- Difficult to provide deep sedation (RASS < -2)
CAUTIONS
- Use caution with patients with depressed blood pressures, albeit can augment with vasopressor support
- Use caution with patients with pulmonary hypertension
- Avoid in patients with severe heart failure, heart block, and bradycardia
Note: Use of benzodiazepines for sedation in patients in the ICU has been shown to exacerbate ICU delirium and increase mortality.20 However, benzodiazepines should be used judiciously and are utilized in specific patient populations (eg, treatment of alcohol withdrawal syndrome, status epilepticus).
Midazolam
Common dosages
- Loading dose: 0.01 to 0.05 mg/kg (0.5 to 4 mg)
- Maintenance infusion rate: 0.02 to 0.1 mg/kg/hr
- Intermittent bolus dose: 0.5 - 4 mg
Mechanism
- Binds to GABA-A receptor complex inducing conformational change in chloride channel. Enhances chloride channel opening frequency resulting in neuronal inhibition.
- Acts on glycine receptors and produces a muscle-relaxing effect
- Onset: 2-5 min
- Duration: 1-4 hrs
Pros
- Dose-dependent, hemodynamically stable medication
- Immediate onset of action
- Anticonvulsant properties
- Amnestic
- Treats alcohol withdrawal syndrome and sympathomimetic intoxication (ie, cocaine)
Cons
- Metabolizes to active metabolites which may cause prolonged sedation
- Deliriogenic
- Risk factor for post-traumatic stress disorder
- May cause paradoxical agitation
- Prone to causing withdrawal
CAUTIONS
- Use caution with elderly patients, due to risk of delirium
Lorazepam
Common dosages
- Loading dose: 0.02-0.04 mg/kg (1-2 mg)
- Maintenance bolus: 0.92 - 0.06 mg/kg q2-6hrs (1-4 mg)
- Infusion rate: 0.01-0.1 mg/kg/hr (max less than 10 mg/hr)
Mechanism
- Binds to GABA-A receptor complex inducing conformational change in chloride channel. Enhances chloride-channel opening frequency resulting in neuronal inhibition
- Onset: 15-20 min
- Duration 6-8 hrs
Pros
- Relatively safe in patients with mild to moderate hepatic and renal impairment
- Anticonvulsant properties
- Amnestic
- Treats alcohol withdrawal syndrome and sympathomimetic intoxication (ie, cocaine)
Cons
- Propylene glycol toxicity
- Deliriogenic
- Relatively slow onset
- Risk of prolonged sedation
- Risk factor for post-traumatic stress disorder
- May cause paradoxical agitation
- Prone to causing withdrawal
CAUTIONS
- Use caution with elderly patients, due to risk of delirium
Diazepam
Common dosages
- Loading dose: 0.05-0.2 mg/kg (5-10 mg)
- Maintenance dose: 0.03-0.1 mg/kg every 0.5-6 hrs
- Continuous infusion not recommended
Mechanism
- Binds to GABA-A receptor complex inducing conformational change in chloride channel. Enhances chloride channel opening frequency resulting in neuronal inhibition.
- Additional specific allosteric binding in the limbic system
- Binds to alpha-2 receptors in the spinal cord
- Onset: IV 2-5 min
- Duration: 20-60 min
Pros
- Rapid onset
- Muscle-relaxant
- Anticonvulsant properties
- Amnestic
- Treats alcohol withdrawal syndrome and sympathomimetic intoxication (ie, cocaine)
Cons
- Deliriogenic
- Propylene glycol toxicity
- Risk of prolonged sedation
- May cause increased prolonged duration for patients with renal impairment
- Risk factor for post-traumatic stress disorder
- May cause paradoxical agitation
- Prone to causing withdrawal
CAUTIONS
- Not typically used in critically ill patients
- Use caution with elderly patients, due to risk of delirium
Acknowledgment
The authoring team wishes to offer special thanks to faculty reviewer Samantha Strickler, DO, FACEP, of Emory University, for her guidance and collaboration.
References
- Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724-731.
- Stephens RJ, Ablordeppey E, Drewry AM, et al. Analgosedation practices and the impact of sedation depth on clinical outcomes among patients requiring mechanical ventilation in the ed: a cohort study. Chest. 2017;152(5):963-971.
- Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39(5):910-918.
- Degrado JR, Anger KE, Szumita PM, Pierce CD, Massaro AF. Evaluation of a local ICU sedation guideline on goal-directed administration of sedatives and analgesics. J Pain Res. 2011;4:127-134.
- Acheson E. Etomidate: Drug Information. UpToDate.
- Joseph J, DiCorpo JE, Rice D, Merlin MA, Weber A. Succinylcholine vs. Rocuronium: Battle of the RSI Paralytics. JEMS. May 13, 2019.
- Ramos-Matos CF, Bistas KG, Lopez-Ojeda W. Fentanyl. [Updated 2022 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459275/.
- Murphy PB, Bechmann S, Barrett MJ. Morphine. [Updated 2022 Jun 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526115/.
- Abi-Aad KR, Derian A. Hydromorphone. [Updated 2022 Jul 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470393/.
- Farkas J. Internet Book of Critical Care. EmCrit.org. https://emcrit.org/ibcc/toc/.
- Muellejans B, Matthey T, Scholpp J, Schill M. Sedation in the intensive care unit with remifentanil/propofol versus midazolam/fentanyl: a randomised, open-label, pharmacoeconomic trial. Crit Care. 2006;10(3):R91.
- Folino TB, Muco E, Safadi AO, et al. Propofol. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430884/.
- Tietze K, Fuchs B. Sedative-analgesic medications in critically ill adults: Properties, dose regimen, and adverse effects. UptoDate. 2022. Retrieved May 2023 from
- Reel B, Maani CV. Dexmedetomidine. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513303/.
- Lingamchetty TN, Hosseini SA, Saadabadi A. Midazolam. [Updated 2023 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537321/.
- Ghiasi N, Bhansali RK, Marwaha R. Lorazepam. [Updated 2023 Jan 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532890/.
- Dhaliwal JS, Rosani A, Saadabadi A. Diazepam. [Updated 2022 Sep 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537022/.
- Freeman CL, Evans CS, Barrett TW. Managing sedation in the mechanically ventilated emergency department patient: a clinical review. J Am Coll Emerg Physicians Open. 2020;1(3):263-269.
- Stephens RJ, Dettmer MR, Roberts BW, Ablordeppey E, Fowler SA, Kollef MH, Fuller BM. Practice Patterns and Outcomes Associated With Early Sedation Depth in Mechanically Ventilated Patients: A Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(3):471-479.
- Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41(9 Suppl 1):S30-S38.