In patients with multiple comorbidities or severe underlying heart disease, ETI may precipitate cardiac ischemia or dysrhythmia leading to collapse.
A 72-year-old female with a history of atrial fibrillation presents to your emergency department with a fever. A chest X-ray shows a right lower lobe pneumonia. The patient is severely tachypneic and hypoxic. Her oxygen saturation is 90% while on 100% non-rebreather. The monitor shows atrial fibrillation with rapid ventricular response and her blood pressure is steadily dropping despite fluid resuscitation; it is now 80/40. You quickly discuss goals with the patient and family and decide to intubate. You use rapid sequence intubation with 20 mg of etomidate and 100 mg of succinylcholine and place the endotracheal tube successfully on the first pass. However, immediately after intubation the patient becomes increasingly hypotensive, then bradycardic, at which point she arrests”¦.
Introduction
Endotracheal intubation (ETI) is a life-saving procedure used by emergency department physicians to provide definitive oxygenation, ventilation, or airway protection for the severely ill patient. Unfortunately, ETI can precipitate rapid and unexpected decompensation, particularly among ED patients who are at high risk for cardiovascular collapse.1 In fact, 25% of patients develop transient hypotension after emergent ETI2 and there is a 30-fold increase in adverse events among ED intubations compared with those performed in the operating room (OR).3
Emergency department intubations are critically different from OR intubations in two ways. First, an ETI in the OR setting is almost always planned well in advance, allowing time for the patient's gastric contents to empty. Contrast this to an emergency department ETI, which is almost never planned in advance and, thus, patients frequently have (or are presumed to have) a full stomach. Second, patients who require ETI in the ED are by definition acutely ill and are more likely to have hemodynamic instability than those undergoing ETI in the OR, where medical optimization of a patient's condition is sought prior to procedure.
Rapid sequence intubation (RSI) was developed to provide optimal conditions for quick, safe ETI outside of the OR. It comprises three basic elements: pre-treatment, induction, and paralysis.4 RSI is especially helpful in addressing the issue of NPO status because the rapidity of the RSI protocol helps minimize the risk of aspiration. However, RSI does little to protect or prevent post-intubation hemodynamic decompensation. The effective emergency provider must develop his or her own techniques for preventing and responding to post-intubation instability.
Physiology
Post-intubation hypotension is a reflection of changes to one or more factors affecting circulatory physiology: gas exchange, venous return, intrathoracic pressure, and cardiac output.
Gas exchange. Adequate exchange of oxygen and CO2 is critical to cardiovascular functioning. ETI is intended to secure adequate gas exchange in critically ill patients. However, gas exchange is easily imperiled by complications of ETI. Esophageal intubation, bronchial main-stem intubation, tension pneumothorax, inappropriate ventilator settings, and mechanical problems with the ventilator circuit are all potential complications of ETI that affect gas exchange. Each of these complications may ultimately result in precipitous hemodynamic collapse. Fortunately, these problems can be quickly assessed and addressed.
Venous return and intrathoracic pressure. Venous return (VR) is the second critical element of cardiopulmonary physiology, determining cardiac preload and, thus, cardiac output. Simply described, VR is proportional to the difference between extrathoracic and intrathoracic pressure (ITP) — venous blood returning from the venules in the tissue beds must overcome ITP in order to return to the right heart. During spontaneous respirations, ITP is either negative or zero at its peak. However, intubation and positive pressure ventilation (PPV) affect return of venous blood to the heart in several ways.
When patients are placed on PPV, intrathoracic pressure increases above zero, which in turn impedes venous return. This phenomenon may not become clinically relevant in a healthy patient who has plenty of room on the Frank-Starling curve. But in patients whose preload was barely adequate prior to PPV, the decrease in VR and preload may lead to a significant decrease in end-diastolic filling pressure and cardiac output. Furthermore, elevations in ITP are also exacerbated by gas trapping; inadequate attention to ventilator settings for patients intubated with obstructive lung disease (e.g., COPD or asthma) may lead to drastic rises in ITP and critical loss of VR.
Venous return is also affected by the volume of “stressed” venous blood in the system, which in turn is affected by overall volume status of the patient and adrenergic action on the vascular system. Critically ill patients presenting to the emergency department frequently have intravascular volume depletion. These patients maintain their stressed venous volume based on the action of sympathetic hormones on the tone of the peripheral vasculature. During ETI, patients receive sedative-hypnotic medications that counteract this sympathetic drive and lead to relaxation of the vasculature, further decreasing the volume of blood returning to the heart.
Cardiac output. Cardiac output (CO) is affected by multiple factors, which in turn are affected by ETI. Cardiac output is proportional to stroke volume (SV) and heart rate (HR): CO = HR x SV
ETI can decrease both stroke volume and heart rate. Sedative-hypnotic agents may affect chronotropy (HR). For instance, many severely ill patients maintain their CO with compensatory tachycardia. This reflex can be blunted by RSI medications. ETI can also decrease SV by either decreasing VR or inotropy. Venous return is decreased via vasodilation, which leads to decreased end-diastolic volume and SV. Increasing ITP under PPV conditions also increases impedance of VR and decreases CO. Inotropy is also decreased by certain sedative-hypnotic agents, decreasing CO. Specifically, the negative inotropic effects of propofol are well studied. Etomidate is thought to be beneficial in terms of cardiac function, but its solvent (propylene glycol) has slightly negative inotropic effects.5 Most sedative hypnotics may lead to vasodilation by blunting of compensatory adrenergic stimuli and decreasing coronary artery perfusion pressure, which, in turn, can lead to negative inotropy.
Patient factors. In addition to the factors listed above, patient-specific factors have a profound effect on post-intubation hemodynamics — in fact, some clinicians believe it is the comorbidities and not the ETI that are primarily to blame for post-intubation hemodynamic collapse.6
Familiarize yourself with two key patient-specific factors when planning your intubation:
- What is the underlying cause of the patient's respiratory failure — hypoxic, hypoventilatory, metabolic, or airway protection?
- Is there underlying heart or lung disease or underlying volume depletion? These factors all have a considerable effect on cardiovascular response to ETI.7
Management
Post-intubation hypotension has diverse presentations and clinical implications. It is important to take into account the entire clinical picture; quick decisions are required! That being said, avoid springing into action over the numbers on the monitor alone. For example, a young healthy trauma patient may sustain a transient drop in blood pressure after intubation that is likely to be well tolerated and may require minimal intervention beyond IV fluid infusion. However, as demonstrated in the case above, post-intubation hypotension can quickly lead to cardiovascular collapse and cardiac arrest in susceptible patients. Management techniques are considered below, categorized into pre-intubation and post-intubation care.
Pre-intubation. For patients with signs or symptoms of shock prior to intubation, post-intubation hypotension can be common and severe. Avoid assessing shock by blood pressure alone! Be aware that patients in compensated shock may be closer to hemodynamic collapse than their blood pressure initially indicates. Consider the following preventative measures:
- Fluids. Ample fluid resuscitation prior to intubation can help stave off the deleterious effects of vasodilation, dehydration, and decreased VR in the patient with septic or hemorrhagic shock. If time allows, try to bolus the patient with fluid directly prior to intubation. It may be possible to infuse a liter of NS while setting up for intubation if you have adequate peripheral access or a pressure bag.
- Vasopressors. In patients who are hypotensive prior to intubation (or those hovering near hypotension), consider preparing and hanging a vasopressor before the intubation. Ideally, pressors are only initiated after adequate fluid resuscitation. In reality, there are clinical scenarios that may not afford you time to complete fluid resuscitation prior to ETI. For instance, it is not uncommon for a patient to present in a mix of cardiogenic and septic shock. These patients may be suffering from severe pulmonary edema in addition to sepsis, making it difficult to adequately resuscitate without airway protection.Some clinicians may choose to start vasopressors prior to intubation on a case-by-case basis, while others may find it helpful to have the pressor hanging for immediate use after intubation. The logistics of initiating vasopressors can be time consuming. Depending on your shop and nursing availability, if you wait to order vasopressors until after you see post-intubation hypotension, your patient may suffer serious decompensation while the medication is being retrieved, pumps programmed, etc. In patients presenting with septic shock, consider selecting your pressor based on the surviving sepsis protocols.
- “Push-dose pressors” (PDPs). PDPs, also known as bolus-dose pressors, have been increasingly used as a treatment for post-intubation hypotension and have received a lot of press in EM educational outlets. In theory, PDPs are similar to drip pressors, except they are tailored for quick-on, quick-off indications. The concept of using a pressor in small bolus form for the treatment of hypotension after ETI was borrowed from the anesthesia literature. While the use of bolus-dose pressors is widespread in anesthesia practice (typically boluses of epinephrine and phenylephrine), the literature to date is mostly derived from OB anesthesia, where PDPs are used to reverse transient hypotension after spinal anesthesia during Cesarean delivery.8,9 Critics of PDPs are concerned that the safety and efficacy in critically ill ED patients is unproven and recommend careful use in appropriate clinical scenarios. Keep your eyes open for more information as this debate evolves.10,11
- RSI medications. In the patient who is hypotensive prior to intubation, or hovering near hypotension, consider avoiding vasodilator induction agents, such as propofol or midazolam.12 Ketamine is frequently cited as the drug of choice for hypotensive patients.13,14 Some experts have recommended using reduced doses of sedative-hypnotic agents in patients who are already in compensated or decompensated shock, regardless of whether the medication is “hemodynamically stable.”15 This may mean trying 10 mg instead of 20 mg of etomidate. Always have the complete dose drawn up in case the initial dose is not sufficient. Finally, don't confuse reduced dosing of sedative-hypnotics with reduced dosing of paralytics. Paralytics improve your chances of first-pass success and, in critically ill patients, first-pass success is paramount.
Post-Intubation
When assessing post-intubation hemodynamic collapse, you must consider each of the above factors simultaneously: gas exchange, venous return, and cardiac output, as well as patient-specific factors. Perform a rapid assessment of your patient and, if possible, simultaneously recruit help from your team. Can the respiratory therapist detach the ventilator and listen for exhale or assess compliance? Can your nursing colleagues ensure adequate fluid resuscitation and prepare meds? Act in parallel; not in sequence!
- Problem — inadequate gas exchange. Your only indications of poor gas exchange may be hypoxia, hypercapnia, or change in breath sounds. Steal a moment to evaluate your patient, monitor, and ventilator for clues (this means being familiar with the layout of your vent screen). Are you seeing a change in O2 or CO2 on the monitor? Are the ventilator settings concerning (e.g., low tidal volumes or high peak pressures)? In this case, gas exchange may be the primary issue. Consider using the mnemonic “DOPES” to troubleshoot a patient with post-intubation hemodynamic collapse. DOPES helps cue the EM physician to readily fixable causes of post-intubation complications (Table 1).
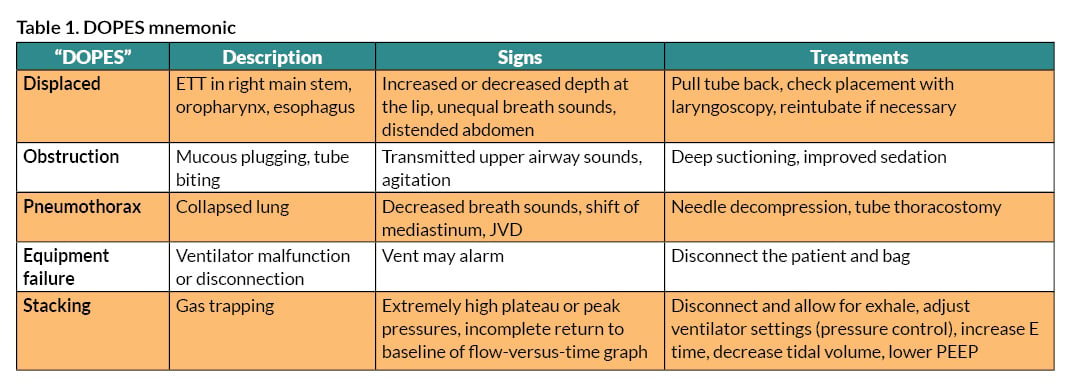 Following the mnemonic, start by disconnecting the patient from the ventilator and perform bag-valve-mask ventilation. This eliminates the chances that the circuit is the problem and helps gauge lung compliance. Second, check the airway: Has the tube been displaced from its initial marking? If it is too deep (main-stem) or too shallow (oropharynx), the tube placement will need to be addressed immediately. Next, check for equal breath sounds and good end-tidal CO2. If there is concern for pneumothorax, decompress immediately. Listen for the quality of breath sounds. If wheezing has developed, this may represent severe bronchospasm, underlying parenchymal disease, or ARDS and may require adjustments to the ventilator. Use deep suction to assess for obstruction due to mucous plugging. Poorly sedated patients may be biting the tube and causing obstruction of airflow.
Following the mnemonic, start by disconnecting the patient from the ventilator and perform bag-valve-mask ventilation. This eliminates the chances that the circuit is the problem and helps gauge lung compliance. Second, check the airway: Has the tube been displaced from its initial marking? If it is too deep (main-stem) or too shallow (oropharynx), the tube placement will need to be addressed immediately. Next, check for equal breath sounds and good end-tidal CO2. If there is concern for pneumothorax, decompress immediately. Listen for the quality of breath sounds. If wheezing has developed, this may represent severe bronchospasm, underlying parenchymal disease, or ARDS and may require adjustments to the ventilator. Use deep suction to assess for obstruction due to mucous plugging. Poorly sedated patients may be biting the tube and causing obstruction of airflow. - Problem — decreased cardiac output.
Inadequate CO can lead to cardiovascular collapse and decreased tissue perfusion of vital organs such as the brain, heart, gut, and kidneys. Signs may be immediately obvious (hypotension, cardiac rhythm change on the monitor from poorly perfused coronaries) or more subtle (skin mottling, change in capillary refill). Unless you have started high-dose vasopressors, hypotension and decreased perfusion is likely caused by changes in VR or HR. Patient-specific factors will help guide your workup. In patients with septic or hemorrhagic shock, consider fluid and pressor management as described above under “pre-intubation.” For patients with underlying obstructive lung disease, always rule out tension pneumothorax, breath stacking, and barotrauma first ”“ each entity can drive up ITP and decrease VR and CO. In patients with multiple comorbidities or severe underlying heart disease, ETI may precipitate cardiac ischemia or dysrhythmia leading to collapse. Evaluate the rhythm on the monitor, obtain a 12 lead EKG, and assess HR and signs of perfusion. In this case, your management will depend on underlying rhythm, presence of a pulse, and evidence of ischemia. - Problem — adequate sedation.
In some cases, your patients may narrowly dodge adverse outcomes during RSI, only to develop hemodynamic collapse when post-intubation sedation is initiated. While it is critical to be on the lookout for this complication of sedation packages, do not be lured into thinking your patient must do without sedation. Be creative! It may be necessary to avoid protocoled sedation packages that will endanger your patient. Be sure to talk to your nurse and develop a plan before you leave the room.Ketamine may be a good resource. While commonly used as an RSI medication, ketamine can also be used in the sedation package. If your patient is thrashing and waking up from ETI, consider starting with a bolus of 1-2 mg/kg IV then a drip at 0.5 mg/kg/hr. This may be a strategy that you need to discuss with nursing and pharmacy staff at your hospital first. Fentanyl is also your friend for these patients. It has a quick onset and short duration of action, allowing you to pause if MAPs are looking low. Fluid boluses and even drip pressors may be a necessary adjunct to keeping your patient adequately sedated (as opposed to leaving the sedation off to avoid pressors, with a thrashing and miserable patient).
Conclusion
While definitive evidence has yet to be supplied, preventative measures, high clinical suspicion, and patient-specific adaptations of intubation technique and care are likely to minimize the degree and frequency of post-intubation hemodynamic instability.
REFERENCES
- Bowles TM, Freshwater-Turner DA, Janssen DJ, et al. Out-of-theatre tracheal intubation: prospective multicentre study of clinical practice and adverse events. Br J Anaesth. 2011;107(5): 687-692.
- Franklin C, Jacob S, Hu T. Life-threatening hypotension associated with emergency intubation and the initiation of mechanical ventilation. Am J Emerg Med. 1994;12:
425”“428. - Woodall N, Frerk C, Cook TM. Can we make airway management (even) safer? Lessons from national audit. Anaesthesia. 2011;66 (2):27-33.
- Stept WJ, Safar P. Rapid induction/intubation for prevention of gastric-content aspiration. Anesth Analg. 1970;49:633”“636.
- Atlee, J. L. (2007). Complications in anesthesia. Philadelphia: Elsevier/Saunders.
- Heffner AC et al. Predictors of the complication of post intubation hypotension during emergency airway management. J Crit Care. 2012 Dec;27(6):587-93.
- Heffner C, Swords D, Neale M, et al. Incidence and factors associated with cardiac arrest complicating emergency airway management. Resuscitation. 2013 Nov;84(11):1500-4.
- Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia. 2002 Apr;94(4):920-6.
- Ngan Kee WD, Khaw KS, Lau TK, et al. Randomized double-blinded comparison of phenylephrine vs ephedrine for maintaining blood pressure during spinal Anaesthesia for non-elective caesarean section. Anaesthesia. 2008 Dec;63(12):1319-1326.
- Weingart S. EMCrit Podcast 6 ”“ Push-Dose Pressors. EMCrit Blog. Retrieved December 18, 2014.
- Weingart S, Mattu A, EMRAP Podcast April 2013 ”“ Push Dose Pressors: RANT! And Response. EMRAP. Retrieved Dec. 18, 2014
- Davis DP, Kimbro TA, Vilke GM. The use of midazolam for prehospital rapid-sequence intubation may be associated with a dose-related increase in hypotension. Prehosp Emerg Care, 2001;5:163”“8.
- Morris C, Perris A, Klein J, et al. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009 May;64(5):532-9.
- Jabre P, Combes X, Lapostolle F, et al; KETASED Collaborative Study Group. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomized controlled trial. Lancet. 2009 Jul 25;374(9686):293-300.
- Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005; 101(3):622.