POCUS is a key diagnostic tool that can allow emergency physicians to diagnose cardiac tamponade with the presence of a pericardial effusion with right ventricular collapse during diastole and/or right atrial collapse with systole.
HISTORY OF PRESENT ILLNESS
A 66-year-old female status post single lead implantable cardioverter defibrillator 3 weeks ago, with history of hypertension, hyperlipidemia, and heart failure with reduced ejection fraction (25%) secondary to non-ischemic cardiomyopathy presents to the ED by ambulance with severe epigastric pain, radiating to her back that started 2 hours prior to arrival.
PHYSICAL EXAMINATION
Vitals are: temperature 36.4℃, heart rate 97 bpm, blood pressure 44/23 mmHg, respirations 28/min, oxygen saturation 95% on room air. Examination reveals a lady who is pale, diaphoretic, and anxious-appearing, with a GCS of 14, losing 1 point for verbal. Cardiac and respiratory examination is only significant for diminished lung sounds. She has no peritoneal signs, no focal abdominal tenderness/masses, and gross rectal examination is without blood. The patient is given 1 liter of lactated ringers and 2 units of uncrossmatched blood, resulting in an increase in mean arterial pressure to >65 mmHg and improvement in her mental status. Broad-spectrum antibiotics were also started.
LABS AND IMAGING
The patient’s bedside glucose was 212 mg/dL. Hemoglobin prior to transfusion was 10 g/dL, lactate was 4.2 mg/dL with a mild anion gap metabolic acidosis. Otherwise, labs such as the comprehensive metabolic panel, coagulation panel, high sensitivity troponin, and brain natriuretic peptide were unremarkable. A computed tomography angiography (CTA) of her aorta through the chest/abdomen/pelvis (Figure 1) was emergently obtained, which revealed a pacemaker lead perforation through the right ventricle with resulting hemopericardium. The patient's blood pressure continued to decline, prompting 2 more units of uncrossmatched blood, norepinephrine, and vasopressin to maintain MAP goals.
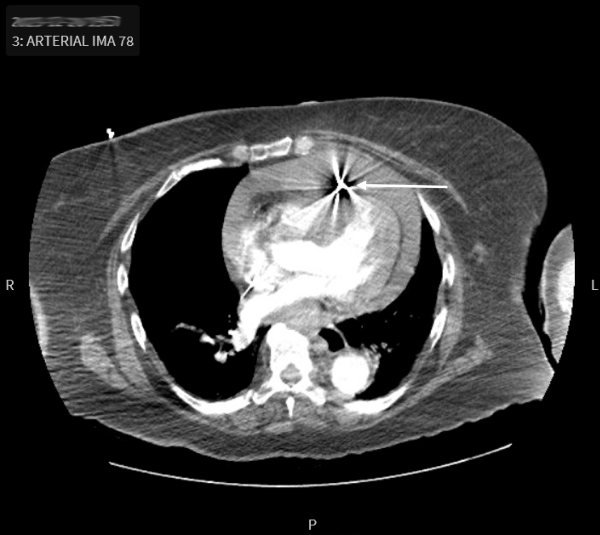
Figure 1. CTA chest axial with pacemaker lead perforation (white arrow) through the anterior right ventricle wall and hemopericardium
POCUS
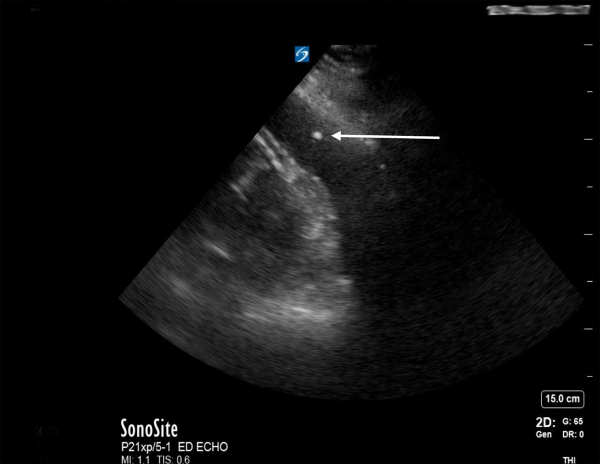
While emergent cardiac surgery consultation was being obtained, the ED team performed a parasternal-approach, ultrasound-guided ED pericardiocentesis (Figure 2) under procedural sedation with ketamine. A subxiphoid approach for pericardiocentesis was deferred as the patient had a large liver cyst obstructing the path and the largest fluid pocket was visualized in the parasternal long view. The removal of 90 mL of sanguinous fluid immediately stabilized the patient’s vital signs, improved mental status, and ultimately negated the need for vasopressors.
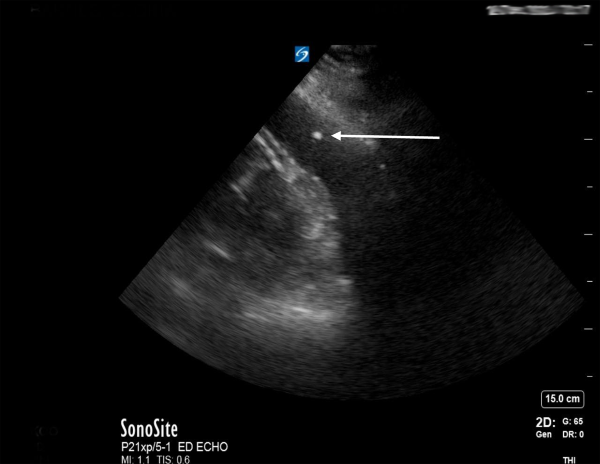
Figure 2. Bedside echocardiogram with spinal needle tip (white arrow) visualized in the hemopericardium during therapeutic pericardiocentesis
DISCUSSION
The patient had cardiac tamponade secondary to hemopericardium from pacemaker and defibrillator lead right ventricular free wall perforation. Lead perforation is considered a rare complication with a majority occurring within the first year of implantation and the incidence rate is estimated to be approximately 0.1%-1%.1,2 Lead perforation of the myocardium can lead to life-threatening complications such as cardiac tamponade.
POCUS is a key diagnostic tool that can allow emergency physicians to diagnose cardiac tamponade with the presence of a pericardial effusion with right ventricular collapse during diastole and/or right atrial collapse with systole. There can be other findings on echocardiogram to include a plethoric inferior vena cava (>20 mm), which can be up to 92% sensitive, but not necessarily specific.4 Also, septal “bounce,” described as intraventricular septal deviation towards the left ventricle during inspiration can be another finding of tamponade physiology. In an emergent setting with hemodynamic instability, an ED pericardiocentesis can be performed to allow ventricular filling. With ultrasound-guidance, major complication rates of pericardiocentesis can be lowered from 25% to 3%.4-6
HOSPITAL COURSE
The cardiac surgery team performed a sternotomy and pericardiotomy, revealing a lead perforation of the right ventricle and an innominate vein injury (Figure 3). The lead was removed and repair of the ventricle and vein performed. The hospital course was prolonged with the complication of a sternal wound infection, but the patient improved on intravenous antibiotics and was discharged home with a wearable defibrillator. She attended her outpatient follow-up appointments with cardiac surgery and cardiology without complications.
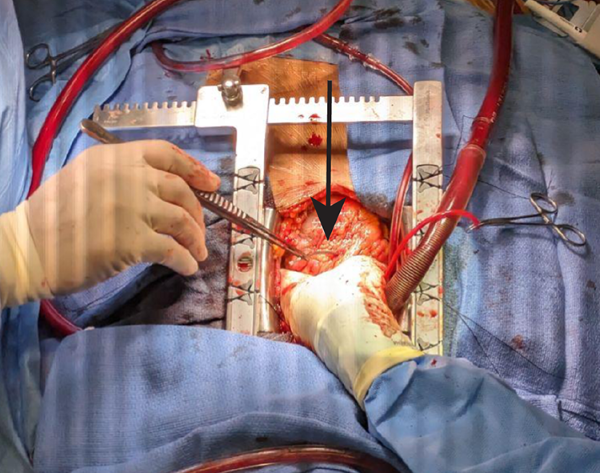
Figure 3. Intraoperative photograph of perforating pacemaker lead, grasped by forceps (arrow)
REFERENCES
- Simsolo E, Wilkoff BL. A Shocking Case of Pacemaker Lead Perforation. JACC Case Rep. 2022 Sep 21;4(18):1203-1205.
- Migliore F, Zorzi A, Bertaglia E, et al. Incidence, management, and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin Electrophysiol. 2014 Dec;37(12):1602-1609.
- Jacob S, Sebastian JC, Cherian PK, Abraham A, John SK. Pericardial effusion impending tamponade:a look beyond Beck’s triad. Am J Emerg Med. 2009;27(2):216-219.
- Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. Echocardiographic Evaluation of Pericardial Effusion and Cardiac Tamponade. Front Pediatr. 2017;5:79.
- Nitta M, Takano K, Yamanaka S. Precautions When Performing Pericardiocentesis in Patients with Cardiac Tamponade-complicated Malignancy: A Case Report and Review of the Literature. Intern Med. 2023;62(9):1311-1317.
- Maggiolini S, Gentile G, Farina A, et al. Safety, efficacy, and complications of pericardiocentesis by real-time echo-monitored procedure. Am J Cardiol. 2016;117(8):1369–74.