Case 1: A 4-year-old girl presents to the ED with nausea and vomiting. The family notes that for the past week the patient has been more tired than usual and having decreased appetite. She has had 3 days of runny nose, cough, vomiting, and abdominal pain. Her vitals are T: 38.7, BP: 95/60, HR: 123, RR: 35, O2 sat: 97%. On exam, you notice that the patient is sleepy-appearing, but arousable. Her abdomen is soft without focal tenderness. She is tachypneic and taking deep breaths.
Case 2: A 14-month old toddler presents to the ED with fever. His dad reports that the patient has had fever, cough, and vomiting for one week. Today, the patient has been sleeping all day and breathing heavily. The patient’s vitals are T: 39, BP: 75/40, HR: 145, RR: 40, O2 sat: 91%. On exam, you notice the patient is lethargic, tachypneic, and not responding to painful stimuli.
Case 3: A 15-year old girl with history of diabetes on an insulin pump presents to the ED with vomiting and a syncopal episode. She was at a family party when she collapsed. There was no head trauma or seizure-like activity. Over the past 2 days, she had been intermittently vomiting with some abdominal discomfort and had told her mother her blood sugar readings ranged between 150-250 mg/dL. The patient’s vitals are: T: 37.5, BP: 100/67, HR: 120, RR: 32, O2 sat: 98%. On exam, you see a talkative teenager with a soft, non-tender abdomen, an insulin pump in the lower abdomen, and no signs of trauma. Her urine pregnancy test is negative and her EKG shows sinus tachycardia.
Epidemiology and Pathophysiology
Diabetic ketoacidosis (DKA) is a severe complication of diabetes. Approximately 30% of children in the United States present in DKA at the initial time of diagnosis of type 1 diabetes, and commonly present in this state to the ED.1 Although less common, DKA may also occur in children with type 2 diabetes. DKA tends to be precipitated by factors such as concurrent infection, particularly if the patient is affected by vomiting and dehydration. It may also be precipitated by missed insulin doses or social factors such as alcohol or illicit drug use.
In DKA, insulin deficiency leads to hyperglycemia. Without insulin, cells and tissues are unable to take up and use blood glucose as an energy source. Instead, adipose tissue gets broken down into free fatty acids that are converted into ketoacids by the liver, causing a metabolic acidosis. The presence of high extracellular hydrogen ions causes potassium to shift out of cells into the blood. Potassium is lost due to hyperglycemia driven osmotic diuresis, causing patients to develop a depletion of total body potassium.2 Overall, a counterregulatory hormonal stress response occurs with subsequent worsening insulin resistance, hyperglycemia, and hypovolemia.
Signs and Symptoms
The presentation of a child in DKA can be very insidious, especially those less than 5 years of age who tend to present with a delayed diagnosis of severe DKA. Polyuria and polydipsia may not be appreciated at this age. Initially, the diagnosis of DKA may be delayed or missed in infants or toddlers presenting with concurrent infections such as pneumonia or bronchiolitis, causing the duration of their symptoms to be even longer and putting them at risk of severe acidosis, dehydration, and altered mental status. They may present with increased irritability, weight loss, and physical signs of hypovolemia. A severe Candida diaper rash can be a leading clue towards a diagnosis of diabetes.3
Children older than 5 years old tend to present with polyuria, polydipsia, and vomiting that leads to dehydration. They can also present with weight loss. Many may have non-specific symptoms such as nausea, vomiting, and abdominal pain that could be misinterpreted as appendicitis. Children may also exhibit hyperpnea as a compensatory effort to counterbalance their ongoing metabolic acidosis.
On exam, children may show signs of dehydration such as decreased skin turgor and dry mucous membranes. If severely dehydrated, they may exhibit tachycardia, decreased capillary refill, and potentially hypotension. A careful, thorough examination should be done to find a possible inciting cause that may have precipitated this event.
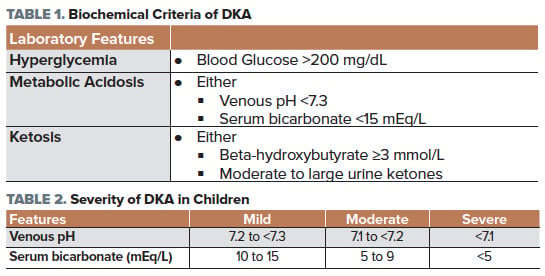
According to the International Society for Pediatric and Adolescent Diabetes (ISPAD) 2018 consensus statement,4 DKA is defined by the presence of all of the following in a patient with diabetes (Table 1).
The severity of a patient with DKA can be determined by a measurement of the venous pH and their serum bicarbonate (Table 2).5
Clinical signs of dehydration tend to be inaccurate, hence markers such as an elevated blood urea nitrogen (BUN) or elevated hematocrit can be helpful.
Management and Treatment
The most important goal in DKA treatment is to restore the body’s glucose regulation back to normal and replace the losses. It is not as simple as giving an insulin bolus because dehydration and a child’s sympathetic activation may interfere with insulin absorption. It’s also critical to address any possible precipitating factors that may have caused a patient to go into DKA. This may include infection, medications, drugs or alcohol, or missed insulin doses in known diabetics.
The management of DKA involves the following 3 steps6:
1. Correct dehydration: Average water losses in children with DKA are around 70cc/kg.7 When calculating fluids, children with mild to moderate DKA and those with severe DKA should be assumed to have 5-7% dehydration and 7-10% dehydration, respectively. The amount of fluids to give to a pediatric patient in DKA is very controversial given the concern of precipitating cerebral edema.8 In general, an initial IV fluid bolus of 20cc/kg of 0.9% normal saline should be given. The goal of initial rehydration is not euvolemia, but to maintain adequate perfusion of end organs. When the patient is hemodynamically stable, judicious replacement of the remaining fluid deficit should be given over 24-48 hours in addition to maintenance fluids. A recent large randomized controlled trial from the Pediatric Emergency Care Applied Research Network (PECARN) showed that neither the rate of fluid administration nor the sodium chloride content of IV fluids significantly affect neurological outcomes in children with DKA during treatment or after recovery.9 However, it must be noted that the range of IV fluid protocols used in the study may have been too narrow to notice a difference.
2. Correct hyperglycemia: Continuous IV insulin infusion should be administered at 0.1 unit/kg/hr. No “bolus” or loading dose is necessary. When the serum glucose reaches 250 mg/dL, dextrose should be added to the IV fluids and insulin should be continued until the ketoacidosis completely resolves. Insulin infusion may be discontinued once the serum anion gap is 12 ± 2 mEq/L or beta-hydroxybutyrate is ≤1 mmol/L, venous pH >7.3 or serum bicarbonate >15 mEq/L, blood glucose <200 mg/dL, and the patient is tolerating oral intake.
3. Correct electrolyte abnormalities: Serum sodium should correct by 1.6 mEq/L for every 100 mg/dL decrease in glucose. Children with DKA have total body deficit of potassium, despite levels appearing to be normal or increased initially. Insulin infusion stimulates intracellular uptake of potassium, putting the patient at risk of hypokalemia. If the patient is hypokalemic (below 3.3 mEq/L), 40 mEq/L of potassium at least should be given and insulin therapy should be delayed as long as possible until potassium levels increase closer to a normal range. If the patient is normokalemic (3.3-5.3 mEq/L) and voiding, 20-30 mEq/L of potassium should be added to the IV fluid maintenance infusions while patient is started on an insulin drip. If the patient is hyperkalemic (above 5.3 mEq/L), potassium should be withheld in the initial fluids. Potassium should be replaced with both potassium phosphate and either potassium chloride or potassium acetate to reduce risk of hyperchloremic metabolic acidosis.2,6 Insulin also stimulates intracellular uptake of serum phosphate leading to hypophosphatemia, putting the patient at risk for rhabdomyolysis, muscle weakness/paralysis, and hemolytic anemia, hence it should also be aggressively repleted. Mild hypocalcemia and hypomagnesemia can occur as well, requiring monitoring and repletion as needed.
PEARL: What needs to be monitored and how often?
Continuously: Vitals, intake and output
Every hour: Glucose (while on insulin), neurologic examinations (including cranial nerves)
Every 2 - 4 hours: Electrolytes and venous blood gas
PEARL: What are the signs of cerebral edema and how can it be treated?
The most concerning complication from treating DKA is cerebral edema. Clinically significant cerebral edema occurs in up to 0.9% of DKA episodes in children, with a mortality rate of 20-25%.10,11 Though rare, children less than 5 years of age with severe DKA are at the highest risk of developing cerebral edema, often due to delayed diagnosis. It is most commonly found in children with severe acidosis or hypocapnia. The key is to treat early. Suspicious signs and symptoms include bradycardia, headache, vomiting, incontinence, fluctuating level of consciousness, posturing, decreasing GCS, abnormal pupillary response, and abnormal respiratory patterns (Cheyne-Stokes, grunting). Treatment of cerebral injury consists of avoiding hypoxia and hypotension, and immediately giving mannitol at 0.5-1 g/kg IV over 15 minutes. This can be repeated in 30 minutes. Hypertonic 3% NaCl at 2.5-5 mL/kg is only reserved as a second line intervention due to one retrospective study that showed higher mortality rates in patients treated with hypertonic saline versus mannitol.12
PEARL: Should a Head CT scan be done to confirm cerebral edema prior to treating?
No. Cerebral edema is a clinical diagnosis with high morbidity and mortality. Treatment should not be delayed. In addition, cerebral edema may not be visualized on initial imaging and may only be apparent hours to days later after neurological decline. Imaging may be helpful to find other etiologies for altered mental status, but if there is high suspicion for cerebral edema, treatment should be initiated immediately.
PEARL: Should fluids be withheld for fear of causing cerebral edema?
Administration of IV fluids should not be withheld due to concerns of causing cerebral edema, especially if the patient looks clinically dehydrated. Fluids should be adjusted to maintain a normal blood pressure to optimize cerebral perfusion in all DKA patients with or without concerns for cerebral edema.
PEARL: What if the ketoacidosis does not improve despite insulin and IV fluids?
Look for another cause of persistent metabolic acidosis such as sepsis, or possibly incorrect preparation/administration of the insulin infusion.
PEARL: Should pediatric patients with DKA receive bicarbonate infusion to correct for acidosis?
No. Bicarbonate infusions have been associated with increased risk of cerebral edema and worsening hypokalemia. The rapid correction of acidosis can also decrease the stimulus for hyperventilation and lead to increased carbon dioxide in the brain causing a decrease in cerebral pH as carbon dioxide crosses the blood-brain barrier.11,13 In very rare situations, bicarbonate can be considered, especially if the child is in severe acidosis, hemodynamically unstable, hyperkalemic, or about to go into cardiac arrest.4
Case 1 Conclusion
Your patient’s fingerstick blood sugar level reads 456 mg/dL and you draw a set of blood and urine samples, while starting her on IV hydration. Her pH is 7.1 with a high beta-hydroxybutyrate result, and positive urine ketones. You hear crackles on lung examination and the chest x-ray shows a right middle lobe infiltrate concerning for pneumonia. After an initial bolus of IV fluids, your patient appears more alert, has not vomited, and is watching clips of Baby Shark on her family’s cell phone. You start her on antibiotics, maintenance fluids, and an insulin drip. However, 2 hours later, you find that your patient’s repeat blood sugar drops to 220 mg/dL. At this time, you add D10 to your maintenance fluids while continuing the insulin drip given that the gap has not closed. The glucose levels continue to drop to 150 mg/dL, so you decrease the insulin drip next. One hour later, you find your patient is more shaky and sleepy with concerns for symptomatic hypoglycemia. You stop the insulin drip for 10 minutes and then restart at a lower rate with more dextrose. She is admitted to the ICU for further monitoring and titration of an insulin regimen for newly diagnosed diabetes.
Case 2 Conclusion
You suspect a critically sick child and bring him to a resuscitation room. He gets IV access and his fingerstick glucose is >400 mg/dL with a blood gas showing pH 6.9 and a bicarbonate of <5. You immediately start fluid resuscitation, empiric antibiotics, and an infectious work-up given your concern for septic shock with his fever and hypotension. You are initially worried about meningitis as a cause of his altered mental status, but recall cerebral edema complications in DKA. He is having more difficulty breathing and worsening lethargy with unimproved blood gases, and you have growing concern for herniation. You talk to the family about a definitive airway and start mannitol. You prepare to intubate with the understanding that the apneic pause during intubation may worsen his acidosis. Fortunately, you visualize the airway without difficulty and rapidly intubate him. You ensure that the respiratory rate on the ventilator is set close to the rate that the patient was breathing at prior to being intubated to compensate for metabolic acidosis. The patient is then transferred to the ICU. After one repeat dose of mannitol, continued hydration, electrolyte repletion, and improving blood sugar levels, the patient is extubated after 2 days, with a normal MRI scan. He is diagnosed with type 1 diabetes and transitioned to subcutaneous insulin therapy.
Case 3 Conclusion
After further interviewing, you find the patient had lied to her mother about her blood sugar ranges at home, for fear of needing to go to the hospital. On her blood tests, she had a blood glucose of 649 mg/dL, a pH of 7.1, and a potassium level of 3.0 mEq/L. You turn off her insulin pump and start her on potassium repletion and IV fluids without dextrose. Each hour, her glucose checks and blood gases improve, and she gets started on 1.5 x maintenance IV fluids with dextrose and an insulin infusion at 0.1 units/kg/hour. She is transferred to the ICU. The patient’s insulin pump was interrogated and found to be dysfunctional and her insulin pump gets switched.
References
1. Wolfsdorf J, Glaser N, Sperling MA, American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150-1159.
2. Fleisher G, Ludwig S. DKA chapter. In: Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia: LWW, 2010.
3. Glaser N. Clinical features and diagnosis of diabetic ketoacidosis in children and adolescents. UpToDate 2019.
4. Wolfsdorf J, Glaser N, Agus A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(suppl):155-177.
5. von Oettingen J, Wolfsdorf J, Feldman HA, Rhodes ET. Use of serum bicarbonate to substitute for venous pH in new-onset diabetes. Pediatrics. 2015;136:e371-e177.
6. Glaser N. Treatment and complications of diabetic ketoacidosis in children and adolescents. UpToDate; 2019.
7. Ugale J, Mata A, Meert KL, Sarnaik A. Measured degree of dehydration in children and adolescents with type 1 diabetic ketoacidosis. Pediatric Crit Care Med. 2012;13:e103-e107.
8. Koves I, Leu MG, Spencer S, et al.. Improving Care for Pediatric Diabetic Ketoacidosis. Pediatrics. 2004;134:e848-e856.
9. Kuppermann N, Ghetti S, Schunk JE, et al. Clinical Trial of Fluid Infusion Rates for Pediatric Diabetic Ketoacidosis. N Engl J Med. 2018;378:2275-2287.
10. Lawrence S, Cummings E, Gaboury I, Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146:688-692.
11. Glaser N, Barnett P, Kuppermann N. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264-269.
12. Decourcey D, Steil G, Wypij D, Agus M. Increasing use of hypertonic saline over mannitol in the treatment of symptomatic cerebral edema in pediatric diabetic ketoacidosis: an 11-year retrospective analysis of mortality. Pediatr Crit Care Med. 2013;14:694-700.
13. Bureau MA, Begin R, Berthiaume Y, et al. Cerebral hypoxia from bicarbonate infusion in diabetic acidosis. J Pediatr. 1980;96:968-973.



