Nerve agents are one of the most terrifying weapons of war. With many direct pathophysiologic effects, the psychological terror they instill is often the intent of their use. Emergency medicine providers must be able to recognize and treat victims of such attacks.
Case
A patient presents to your urban emergency department via EMS with shortness of breath, copious secretions, nausea, vomiting, and stool incontinence. His symptoms began abruptly at a concert and progressively worsened. During transport, he develops signs of increased work of breathing, coarse lung sounds, and hypoxia. You then receive a box call from the concert Incident Command regarding multiple casualties en route to your facility with similar symptoms. What do you do next? Who do you call, what procedures need to be initiated?
Introduction
Nerve agents are one of the most terrifying weapons of war. With many direct pathophysiologic effects, the psychological terror they instill is often the intent of their use.1 Often tasteless and colorless, they can be dispersed over a wide area to inflict as many casualties as possible. Although banned from use in conventional war, there have been increased concerns that terrorist organizations or other non-governmental organizations may seek to use these weapons anyway.1 While the United States has fortunately never seen a nerve agent attack on its soil, the possibility remains high. Emergency medicine providers must be able to recognize and treat victims of nerve agent attacks.
The 4 most common nerve agents are tabun (GA), sarin (GB), soman (GD), and cyclosarin (GF). They have the designation “G” because they were initially synthesized by German scientists in the early 20th century.2 Originally, tabun was designed as an organophosphate pesticide. However, its utility as a weapon was quickly realized and the additional agents were developed. These agents are all liquids at room temperature but can easily be aerosolized by either a dispersal device or by an explosive blast. They are all soluble in both fat and water, meaning they can readily be absorbed through the eyes, skin, and respiratory tract. They are all considered non-persistent compounds meaning that they evaporate quickly.2
The second generation of nerve agents with the designation "V" are much more stable and potent compared to the "G" agents and are considered persistent agents.2 They add a sulfur group to the organophosphates thereby making these agents less volatile as well as more fat/oil soluble. They act through direct skin contact and persist in the environment for up to several weeks due to their hydrophobic nature. The most notable example is VX which is highly toxic and can cause death within a few minutes to hours. 2
Pathophysiology
Nerve agents are very similar to organophosphates; they bind to and inhibit acetylcholinesterase. This produces a toxic accumulation of acetylcholine at the peripheral muscarinic, nicotinic receptors as well as the CNS synapses. Additionally, nerve agents appear to activate the NMDA receptors in the brain and inhibit GABA transmission.3
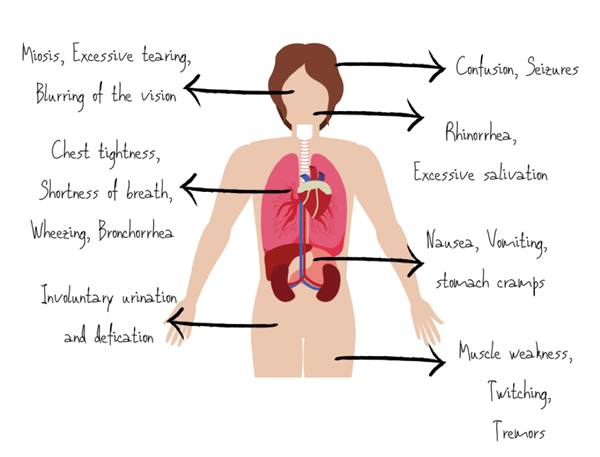
Overstimulation of muscarinic receptors causes miosis, hypersecretion, bronchoconstriction, vomiting, diarrhea, urinary and fecal incontinence, and bradycardia. Over-activating nicotinic receptors in the skin cause sweating, and in skeletal muscle, they cause weakness and flaccid paralysis.3 At CNS cholinergic receptors, nerve agents produce fatigue, lethargy, amnesia, ataxia, seizures, coma, and respiratory depression.3
Symptoms
The clinical presentation of patients exposed to nerve agents depends on the route and duration of exposure. Exposure to lower concentrations of vapor leads to relatively mild symptoms such as miosis, ocular pain and rhinorrhea followed by gastrointestinal and respiratory symptoms with extended durations of exposure.3
Exposure to high concentrations of vapor induces convulsions, flaccid paralysis, loss of consciousness, and ultimately respiratory failure. The severity is due to nerve agent vapor being easily absorbed in the respiratory tract. It is so potent that it exerts its effects within seconds of exposure.3
Treatment
Initial therapy should focus on removing the patient's clothes and decontamination to avoid further skin absorption followed by assessing the patient’s airway, breathing, and circulation. The priority MUST be decontamination.7
Atropine remains the cornerstone of cholinergic toxicity treatment since its mechanism of action works as an acetylcholine receptor antagonist. The dose of atropine is based on the severity of symptoms.4,5
Table 1. Dosing of atropine for cholinergic toxicity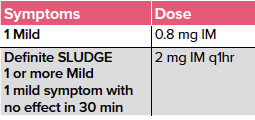
Be aware that you may have to utilize 2-3 times this dose in severe poisoning situations (4-6 mg) titrated to decrease in symptoms.5
SLUDGE: Salivation, Lacrimation, Urination, Defecation, GI upset, Emesis
In addition to atropine, providers should also administer Pralidoxime Chloride (2-PAM chloride). 2-PAM works by reactivating the acetylcholinesterase by scavenging the phosphoryl group and attaching it to the functional hydroxyl group of the acetylcholinesterase.4,5 Delays in the administration of 2-PAM can render it ineffective because of the aging of the agent cholinesterase complex.
TABLE 2. Dosing of 2-PAM for Cholinergic Toxicity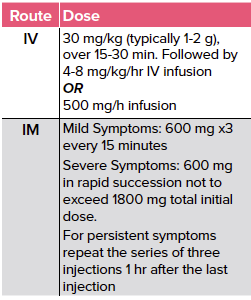
The only class of antiepileptics effective in the management of seizures induced by nerve agents are benzodiazepines. The management of status epilepticus due to nerve agent exposure requires higher doses of anti-epileptic agents than conventional seizure therapy. Animal studies that have been extrapolated to humans estimate that doses as high as 30-40 mg of diazepam may be required to break seizures due to nerve agent exposure.1
Provider Considerations
Your safety and the safety of the ED must be the No. 1 priority during any nerve agent attack. Proper decontamination and disaster planning at a hospital-level must be performed on a regular basis to ensure preparedness for such an event.7
The ED (and hospital) should be immediately locked down with controlled entry and exit once an event has been identified. All emergency notifications to staff and local government agencies should be made. This includes the state health department to begin mobilizing resources.7 The specific level of personal protective equipment (PPE) is dictated by the type of release and air vapor concentrations. At a minimum level, coveralls, gloves, steel toe, shank boots [chemical-resistant], should be used if no air involvement.8 It is unrealistic to expect ED staff to be trained for higher levels of PPE; however, the decontamination group should at minimum be operating in level B PPE (SCBA. Chemical-resistant gloves [double-layered], clothing, steel-toe, and boots) if operating within the hot or warm zone.8 Specific federal decontamination teams are able to be mobilized; however, for the initial incident response, decontamination will fall on the local institution and local emergency response resources.
Personal provider safety is paramount! You cannot treat others if you become a casualty!
Conclusion
Nerve agent attacks have the potential to quickly inflict a high number of casualties. As emergency medicine providers, we will be some of the first providers to treat victims of such an attack. Our ability to recognize and properly treat these patients is paramount to mitigating the damage from such an event. Of course, providers must always be mindful to protect themselves first and foremost.
Take-Home Points
- Symptoms: Salivation, lacrimation, urination, defecation, GI upset, emesis
- Treatment: Decontamination, Atropine and 2-PAM Chloride
- Personal safety and decontamination are the most important considerations for nerve agent attacks
References
- Ganesan K, Raza SK, Vijayaraghavan R. Chemical warfare agents. J Pharm Bioallied Sci. 2010;2(3):166-178.
- Wiercinski A, Jackson JP. Nerve Agents. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020-. 2020 Jan 16.
- Pohanka M. [Cholinesterase activity assays and their use in the diagnosis of various pathological states including poisoning by neurotoxic agents]. Ceska Slov Farm. 2017 Summer;66(4):147-153.
- Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev Med Chem. 2007;7(5):461-466.
- Zorbaz T, Malinak D, Kuca K, Musilek K, Kovarik Z. Butyrylcholinesterase inhibited by nerve agents is efficiently reactivated with chlorinated pyridinium oximes. Chem Biol Interact. 2019;307:16-20.
- Huebner KD, Caneva DC. CBRNE - Nerve Agents, G-series - Tabun, Sarin, Soman Treatment & Management: Approach Considerations, Prehospital Care, Emergency Department Care. Available at: https://emedicine.medscape.com/article/831648-treatment. Published November 9, 2019. Accessed January 27, 2020.
- Tokuda Y, Kikuchi M, Takahashi O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation. 2006;68(2):193-202.