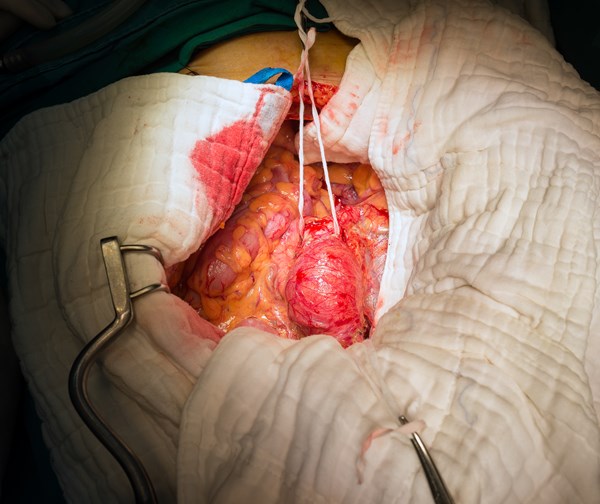
An abdominal aortic aneurysm (AAA), defined as any dilatation of the abdominal aorta greater than 3 cm, is an unfortunately common condition in developed countries.
In the United States, abdominal aortic aneurysms are the 13th leading cause of death. Elderly white males — with associated risk factors of smoking, hypertension, atherosclerotic disease, and family history — are at the highest risk.1 Aneurysms develop as a result of degenerative breakdown of the arterial wall, brought on by a loss in collagen and elastin fibers.2
AAAs classically present with abdominal or flank pain, hypotension or shock, and a pulsatile mass. However, more than half of cases lack one or more of these characteristics, and the first presentation may be the aneurysm rupture itself.3The relatively nonspecific clinical presentation has resulted in AAAs commonly going undetected or being mistaken for other disease processes, leading to an often lethal outcome. Without surgical intervention, which is generally recommended if the aneurysm is greater than 5.5 cm, AAAs can reach almost 100% mortality.
The rising prevalence of bedside ultrasound and implementation of the Rapid Ultrasound for Shock and Hypotension (RUSH) exam in most emergency departments has, fortunately, led to better detection of AAA quickly at bedside.3 Here, we describe a case of a missed ruptured AAA (rAAA), measuring 7.5 cm, in a paraplegic patient not fitting the classic demographic presenting in shock, where the RUSH protocol likely would have reduced delays in care and improved the outcome.
Case
A 54-year-old male with a history of hypertension and T2 paraplegia secondary to a gunshot wound many years ago presented to the ED via ambulance for evaluation of fatigue with associated nausea and decreased appetite. Per patient and paramedics, the fatigue has been ongoing for the past five days. He normally could transfer himself from the bed to the wheelchair, but was unable to do so for the past two days. He also performed intermittent straight catheterizations as a result of chronic urinary retention. Otherwise, the patient denied any fevers, chills, cough, dyspnea, syncope, new focal weakness or numbness, vomiting, diarrhea, or blood in stool. Regarding his T2 paraplegia, he endorsed no sensation or motor function below the T2 vertebral level. He denied any surgical or pertinent family history. He admitted to daily chronic alcohol and tobacco abuse, but no illicit drug use.
On ED presentation, the patient was afebrile, mildly tachycardic at 105 beats per minute, hypotensive with a blood pressure of 80/44 mmHg, and saturating well on room air. Physical examination revealed an alert patient, answering questions and following commands appropriately, with baseline neurologic deficits of lower extremity paralysis and lack of sensation below T2. His abdomen was soft and nondistended without peritoneal signs or palpable masses. The patient’s lower extremity compartments were soft to palpation with 2+ dorsalis pedis pulses.
Given his initial vital signs, the patient was activated for sepsis with our rapid response team. Bedside ultrasound revealed a hyperdynamic left ventricle with complete collapse of the inferior vena cava upon inspiration and absent B-lines on all lung fields. As a result, he was administered 30 mL/kg of intravenous fluids and started on empiric Zosyn and Vancomycin. Status post-administration of intravenous fluids, his blood pressure normalized to 122/70 mmHg, but the patient became bradycardic with a heart rate dropping into the 40s. Given his lack of appetite over several days, there was concern for metabolic/electrolyte derangements which were confirmed by a point-of-care potassium level of 9.2 mmol/L and peaked T waves on electrocardiogram. He was placed on transcutaneous pacer pads and administered medications to temporize his hyperkalemia (calcium gluconate, sodium bicarbonate, albuterol, furosemide, and insulin with dextrose). The patient’s bradycardia was noted to have resolved after these medications as well.
On laboratory analysis, a complete blood count was significant for leukocytosis with a white blood cell count of 27.8 (normal range: 4–10 x 109 per L) and anemia with hemoglobin levels of 8.7 g/dL (normal range: 13–16.5 g/dL). Comprehensive metabolic panel was significant for hyperkalemia of 9 mmol/L (normal range: 3.6–5 mmol/L) as well as acute renal failure with an estimated glomerular filtration rate of 6.23 mL/min/1.73m2 (normal range: ≥ 60 mL/min/1.73m2), creatinine of 9.4 mg/dL (normal range: 0.70–1.3 mg/dL) and BUN of 84 mg/dL (normal range: 7–18 mg/dL).
Due to the patient's bladder scan having revealed no urine output and a repeat potassium at 8.3 mmol/L, the decision was made to consult nephrology for emergent hemodialysis and admit the patient to the medical intensive care unit (MICU) for further management. While boarding in the ED, hemodialysis was initiated overnight.
Once hemodialysis was completed, he was deemed stable to obtain a computed tomography (CT) scan of his abdomen/pelvis to investigate the acute renal failure. After returning from the CT scanner, the patient began to become more tachycardic and borderline hypotensive; nurses also noted faint or absent dorsalis pedis pulses with cool extremities. Radiologists immediately notified our providers that the CT scan revealed a 7.5 cm fusiform abdominal aortic aneurysm (Figures 1, 2, and 3), with surrounding retroperitoneal hemorrhage concerning for abdominal aortic aneurysm rupture. It was suspected that the dialysis worsened the ongoing rupture given his hemodynamic decompensation. The MICU team immediately consulted vascular surgery, and the patient was subsequently taken to the operating room for abdominal endovascular aneurysm repair (EVAR). Although the EVAR was performed successfully, the patient’s right lower extremity was unfortunately unsalvageable secondary to acute limb ischemia and underwent cryoamputation. The patient was hospitalized for the next two months and ultimately discharged to a long-term care facility.
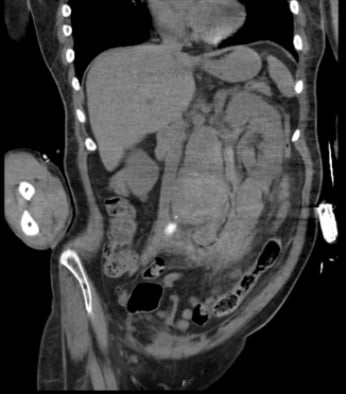
Figure 1
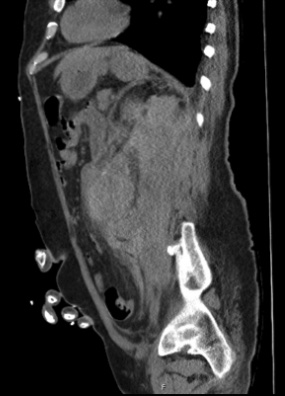
Figure 2
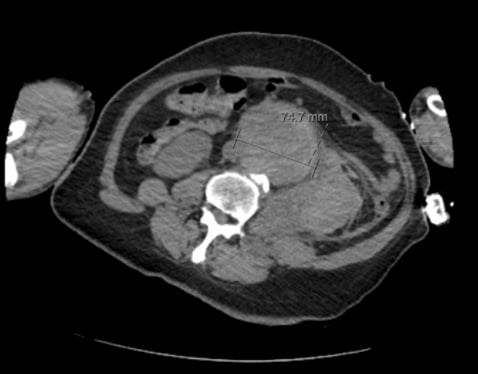
Figure 3
Discussion
An aortic aneurysm is defined as a weakened aortic wall that has dilated by over half of its original diameter, most commonly occurring between the renal and inferior mesenteric arteries.2 The single most important risk factor for developing AAA is a history of smoking, with age greater than 65, being of the male sex, and having family history of AAA following close behind. Additional risk factors include uncontrolled hypertension, coronary artery disease or atherosclerosis, and previous MI.4 While the overall incidence of AAA has been decreasing, attributed in part to improvements in tobacco use and dietary changes, mortality rates secondary to rAAA have remained high at almost 100% without immediate surgical intervention. As aneurysm diameter increases, so does risk of rupture, with 7 cm aneurysms exhibiting a 50% chance of rupture.4
Physical Exam
Although AAA has been documented with a classical presentation of severe abdominal or back pain, hypotension, and a pulsatile abdominal mass, this triad is rarely clinically seen in its entirety.5 Many patients present to the ED only after the aneurysm has ruptured, yet still demonstrate vague and nonspecific symptoms leading to a missed diagnosis of AAA. On physical exam, emergency physicians should assess for pulsatile abdominal masses, increased tenderness over the aneurysm, and signs of lower extremity hypoperfusion such as weakened distal pulses, loss of hair, or non-healing foot wounds.2 A comprehensive physical examination should also include inspection for signs of associated aneurysms including iliac artery aneurysms, which are the most common, and peripheral aneurysms.6
Ultrasound Screening
With sufficient screening and early detection, AAA mortality can be decreased to about 1-2%.4 According to the U.S. Preventive Services Task Force, all men between the ages of 65 and 75 who have ever smoked should undergo a one-time AAA ultrasound screening. Conversely, screening is not recommended for those who have not smoked or for women at all.7 Thus, performing a dedicated ultrasound exam in the ED can offer a noninvasive yet sensitive screening modality for detecting a dilated abdominal aorta in at-risk patients.1
Physical evaluation for AAA in the case of our paraplegic patient was insufficient due to the lack of sensation below the T2 vertebral level. Furthermore, our patient initially presented without any pulsatile mass and had strong distal pulses in the lower extremities. In this instance, the RUSH exam would have saved valuable time in identifying the patient’s AAA prior to obtaining a diagnostic CT. A study by Moore et al reaffirms this by indicating that bedside ultrasound screening by an experienced emergency physician takes no more than 3 minutes of scan time, and measured aneurysm diameter by ultrasound was in line with diagnostic imaging results.1
RUSH Exam
The RUSH exam consists of evaluating the heart, intravascular status, and large arteries in the setting of shock. More specific to this particular case, however, is how the RUSH exam dictates visualization of the abdominal aorta. Following RUSH protocol, emergency physicians can utilize a phased-array or curvilinear transducer in the transverse orientation to visualize the abdominal aorta as a circular vessel anterior to the vertebral column. Moving the transducer from the xiphoid process inferiorly to slightly below the umbilicus, while applying constant pressure to displace bowel gas, will allow for proper visualization of the abdominal aorta. It is imperative to pay special attention to the infrarenal aorta as this is where most AAA are located. While ultrasound can provide valuable insight on the dilation of the aorta, it is limited in its ability to discern whether the aneurysm has ruptured due to the aorta being a retroperitoneal organ.3 In a hemodynamically stable patient, CT and angiography is appropriate to evaluate for leakage of blood. However, hypotensive patients should be assumed to have acute rupture and sent for emergency repair. A study by Kuhn et al revealed that even emergency physicians with limited training and experience in ultrasound techniques can successfully identify the presence — or lack thereof — of AAA, further emphasizing the utility of the RUSH protocol.5
AAA in Setting of Paralysis
Literature review revolving around AAA in patients with previous spinal cord injuries is rather limited. A retrospective study conducted by Jacobs et al through the U.S. Department of Veterans Affairs found that out of 31 patients identified, none presented with symptoms associated with AAA and that the finding of AAA was largely incidental.8Jacobs et al noted that while delayed diagnosis of an AAA could be due to the sensory deficits and the lack of pain perception associated with an aneurysm, patients with sensory-motor deficits are also predisposed to develop complications that would warrant imaging on routine visits. The increased number of abdomen/pelvis imaging and work-up for complications such as nephrolithiasis, urinary tract infections, or pressure sores could play a role in an increased incidental diagnosis of AAA when patients present for unrelated complaints. This retrospective study concluded that AAA repair in patients with longstanding spinal cord injuries can be performed by minimal added mortality. Namely, hemodynamic instability was deemed to not be problematic as these patients present with a compensated degree of lower extremity vasodilation.8 Furthermore, the lack of pain perception in patients such as those identified by Jacobs et al, and in our own case, serves to reinforce the utility of the RUSH exam as a valuable tool in risk-stratifying AAA.
Take-Home Points
- AAAs are generally asymptomatic before rupture and often lethal due to delays in diagnosis and care, as most are missed for alternative diagnoses before hemodynamic compromise occurs.
- Traditional physical exam findings can be unreliable in diagnosing AAA ruptures; this was the case with our paraplegic patient who had no sensation below the T2 level.
- The RUSH exam is a rapid ultrasound protocol that can be easily performed by emergency physicians for evaluation of undifferentiated shock.
- CT scans are the imaging modality of choice, but ED ultrasound is readily available and has high sensitivity and specificity in diagnosing AAA. This may reduce delays in care, especially in the setting of hemodynamic instability.
- Most AAA cases rupture into the retroperitoneal space, making ultrasound less useful in identifying AAA after rupture.
References
- Moore CL, Holliday RS, Hwang JQ, Osborne MR. Screening for abdominal aortic aneurysm in asymptomatic at-risk patients using emergency ultrasound. The American Journal of Emergency Medicine. 2008;26(8):883-887.
- Calero A, Illig KA. Overview of aortic aneurysm management in the endovascular era. Seminars in Vascular Surgery. 2016;29(1-2):3-17.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH Exam 2012: Rapid Ultrasound in Shock in the Evaluation of the Critically Ill Patient. Ultrasound Clinics. 2012;7(2):255-278.
- Keisler B, Carter C. Abdominal Aortic Aneurysm. American Academy of Family Physicians. April 2015:538-543. doi:91(8):538- 543
- Kuhn M, Bonnin RLL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: Accessible, accurate, and advantageous. Annals of Emergency Medicine. 2000;36(3):219-223.
- Shaw PM, Loree J, Gibbons RC. Abdominal Aortic Aneurysm. National Center for Biotechnology Information.Published January 24, 2022. Accessed February 27, 2022.
- Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm. JAMA. 2019;322(22):2219.
- Jacobs DL, Peterson GJ, McKirgan LW, Virgo KS, Johnson FE. Outcome of abdominal aortic aneurysm repair in patients with previous spinal cord injury in the Department of Veterans' Affairs Hospitals. Cardiovascular Surgery. 1997;5(3):286-290.