In 2022, a record number of migrants arrived at the southern border of the United States.1 Many of them traveled through regions of Central America where neglected tropical diseases such as malaria and dengue are endemic.2
Malaria is a mosquito-borne protozoal disease that commonly presents with cyclical fevers and myalgias and can manifest with profound anemia.3 Dengue is a mosquito-borne arbovirus commonly presenting with fevers, myalgias, rash, and retro-orbital pain.4
In 2018, the most recent year for which data is available, the CDC reported 1,823 malaria cases diagnosed in the United States. Many of these cases were in travelers from West Africa.5
Here, we present the case of a migrant from Venezuela with malaria, dengue, and COVID-19 co-infection.
Case Description
A 35-year-old Venezuelan man presented to the emergency department via EMS. He reported feeling generalized weakness and fatigue for 2.5 months. The patient had traveled on foot from Venezuela through Central America and Mexico. During his journey, the patient traveled through the Darien Gap, a large jungle between Colombia and Panama. He reported that physicians in Panama treated him for dengue fever with an unknown injection; he had brief improvement in symptoms, but the symptoms quickly returned.
The patient presented to the ED 6 days after crossing the U.S.-Mexico border, with complaints of generalized body aches, headache, fevers, and darkened urine since his journey began 2 months prior.
On presentation, the patient was hemodynamically stable but febrile with a body temperature of 104.4 degrees Fahrenheit and tachycardic with a heart rate of 149 beats per minute. On physical exam, the patient appeared ill and diaphoretic but could speak coherently in complete sentences. He was jaundiced; however, no rash was present. The patient’s abdomen was soft and non-tender without palpable hepatosplenomegaly. His lungs were clear to auscultation bilaterally, and he remained tachycardic with a sinus rhythm. A tourniquet test for dengue fever was performed by inflating a blood pressure cuff over the patient’s arm for approximately 5 minutes. No petechiae were observed following the test, and it was considered negative.
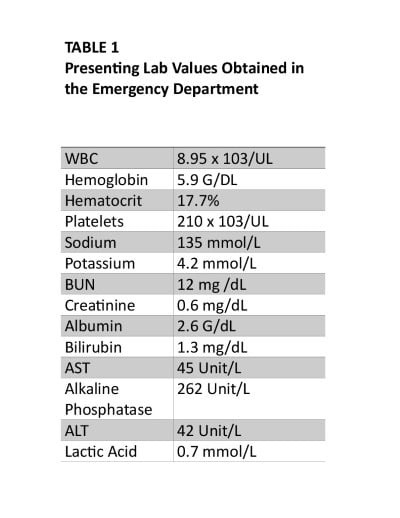
Laboratory studies were remarkable for severe normocytic anemia (hemoglobin 5.9 g/dL), hyperbilirubinemia (total bilirubin 2.1 mg/dL), and a positive COVID-19 rapid antigen test. The patient also had an elevated alkaline phosphatase (262 u/L), but AST/ALT were within normal limits. The patient did not have leukocytosis (Table 1). The initial chest X-ray was unremarkable and not concerning for infectious pneumonia.
The patient was admitted to the hospital to manage presumed plasmodium and dengue virus infection. He had persistent fevers and tachycardia despite antipyretics and IV fluid resuscitation. Malaria and dengue viral studies were pending at the time of admission.
On day one of admission, Plasmodium ring-form trophozoites were identified on a blood smear, and a 3-day course of atovaquone-proguanil was initiated. The patient reported improved symptoms following the initiation of antimalarials, and tachycardia and fever began to improve. By the completion of his antimalarial course, the patient reported that his symptoms had resolved, and he remained asymptomatic for the remainder of his hospitalization.
Plasmodium vivax was eventually detected on PCR, and the patient was discharged on 14 days of oral primaquine after G6PD levels were normal. Additionally, the patient tested positive for IgG dengue viral antibodies during his hospital admission. Hemoglobin improved to 9.3 on the day of discharge without administering blood products. The patient was discharged after several days with improved symptoms and clinical condition.
Discussion
Migrant patients presenting with fever can represent a diagnostic challenge. Migrants from Central and South America often traverse regions where neglected tropical diseases are endemic. The recent influx of migrants from South and Central America means that clinicians should have a high suspicion for tropical diseases in migrant patients presenting with fever and weakness.
In this case, the patient presented with a suspected co-infection of Plasmodium vivax malaria, dengue fever, and COVID-19 — infectious diseases with overlapping symptoms.
Malaria is a protozoal disease caused by Plasmodium sp. It is spread through the bites of infected Anopheles mosquitoes. Malaria is endemic to tropical regions of South and Central America, sub-Saharan Africa, and Asia, having been eradicated from the United States and Europe.3 Plasmodium vivax is the most common malaria species in South and Central America, accounting for most cases.6,7 Plasmodium falciparum is also endemic to this region, but is less common. Falciparum is typically responsible for more severe manifestations of malaria, including cerebral malaria, which can result in seizures, coma, and death.3
Uncomplicated vivax and falciparum malaria manifest as cyclical fevers, myalgias, and chills. Laboratory manifestations include hyperbilirubinemia, thrombocytopenia, and anemia.3
Treatment depends on species and severity. Severe malaria, regardless of species, is typically treated with intravenous artesunate. Uncomplicated non-Falciparum malaria can be treated with oral chloroquine or an artemisinin-based combination therapy (ACT). Due to increasing resistance levels, chloroquine should be avoided when Falciparuminfection is suspected, or the species is unknown. Three days of ACT is the first-line treatment for Falciparum malaria. It is safe and well tolerated in children and adults (after the first trimester in pregnant patients). It is rapidly effective in reducing parasite burden and improving symptoms, as seen in this case. Treatment should not be delayed when malaria is suspected. Second-line treatments include different ACT regimens or 7-day treatments with quinine or artesunate with doxycycline or clindamycin.3
Plasmodium vivax has a dormant hepatic stage (hypnozoite) that can lead to relapses over a year after initial infection. Patients infected with Plasmodium vivax malaria require 14 days of primaquine to eliminate the hypnozoite stage and prevent relapse. However, patients must be tested for G6PD deficiency before initiation of primaquine because this medication can induce hemolysis in patients with G6PD deficiency. Lower dosing of primaquine can be used for these individuals.3
Dengue fever is an arbovirus transmitted by the Aedes mosquito. Its distribution significantly overlaps with malaria and is endemic to Central and South America, sub-Saharan Africa, and Asia. Unlike the Anopheles mosquito, Aedes sp.is also found in the southern United States and serves as a vector for dengue virus, zika virus, chikungunya, and yellow fever.14
Because Aedes sp. is endemic to the United States, local transmission of dengue fever can occur. However, most cases of dengue fever are imported by travelers. In 2022, 1,188 cases of dengue fever were reported to the CDC; of these, only 4.9% were suspected to be locally transmitted.15
Dengue classically presents with fever, retro-orbital headache, and severe myalgias. A macular, erythematous rash is sometimes present during the end of the febrile period. Most episodes of dengue resolve within 5-7 days. Laboratory abnormalities include leukopenia and thrombocytopenia.4 More severe manifestations of dengue include dengue hemorrhagic fever, which is notable for severe coagulopathy and hemorrhage.4
Given this patient’s rapid clinical improvement following ACT, it is likely that Plasmodium vivax infection was the primary source of his symptoms. The patient’s positive IgG antibody to dengue virus likely represented a more remote infection, plausibly at the start of his journey to the United States. IgM and the dengue NS1 antigen were not detected. Both the NS1 antigen and IgM antibody to dengue are markers of recent infection. The NS1 antigen is detectable from days 1-18 following infection, and the IgM antibody is detectable 5 days after infection and can persist for up to 3 months following infection. IgG will typically be detectable for years following infection.16
Based on the patient’s recent reporting of a dengue viral diagnosis, we presume he likely acquired a dengue viral infection that was then closely followed or overlapped by subsequent malaria infection. The patient reported frequently being bitten by mosquitoes while crossing the Darien Gap, and he likely contracted malaria and dengue during this period. Both Aedes and Anopheles mosquitoes are common in the Darien jungle, as well as in Central America.8,9 His dengue fever likely self-resolved prior to admission. The time since infection (more than 2 months) likely contributed to a heavy parasite load and was responsible for the severity of his symptoms, including profound anemia.
Unfortunately, this patient’s hospital stay was prolonged, pending the PCR identification of the malaria species responsible for his infection to determine whether primaquine was necessary. Hospitals treating migrant patients may consider utilizing malaria rapid diagnostic tests (RDTs) to prevent delays in care. The malaria RDT, approved for use in the United States, has a sensitivity and specificity of 84.2% and 99.8%, respectively.10 Malaria RDTs can differentiate between Falciparum and non-Falciparum species of malaria.10
It is unclear to what degree COVID-19 contributed to the patient’s presentation. Given his rapid improvement following antimalarials, he likely had an asymptomatic or mild COVID- 19 infection. Treatment with Paxlovid was deferred as it was unknown when the patient contracted COVID-19, and Paxlovid has significant interactions with atovaquone.17
Prior to arrival, the patient was staying at a shelter. Many migrants initially rely on shelters for housing after crossing into the United States. These shelters, often run by non-governmental organizations, are frequently over maximum capacity with poor infection-control measures. COVID-19 should be considered in any migrant patient presenting with a fever.
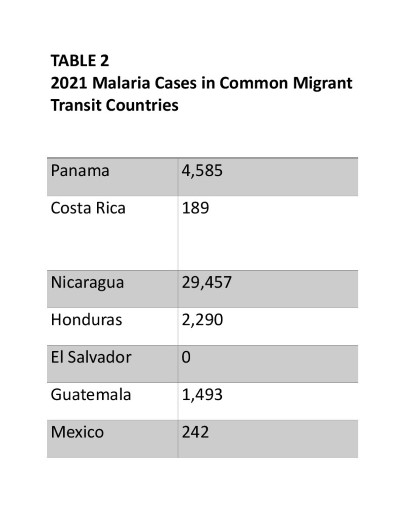
The Central American tropical lowlands, through which migrants traverse enroute to the United States, are host to many endemic tropical diseases. Malaria is endemic in each country migrants commonly transit through, except for El Salvador (Table 2).18 Areas of particularly high incidence along the transit route include southern Panama and the Caribbean coasts of Nicaragua and Honduras.
This patient became symptomatic after traveling through the Darien Gap, a jungle where migrants sleep outside, are constantly exposed to mosquitos, and drink dirty stream water. Mosquito-borne arboviruses, such as dengue, chikungunya, and zika, are increasingly concerning in this region.11 The prevalence of dengue in Central America increased by 288% from 2000 to 2017.12
In the border community of El Paso, Texas, where this patient was treated, malaria cases are increasing. From 2018-2021, 3 cases of malaria were reported. In 2022 and the first several months of 2023, 19 malaria cases were reported, a 533% increase.
As more migrant patients travel to the United States through Central America, clinicians must be able to recognize the tropical diseases they are exposed to during their journey.
Conclusion
In 2022, U.S. Customs and Border Protection reported 2.76 million encounters with migrants at the southern border of the United States.1 A common route for migration is through the tropical lowlands of Central America, including the Darien Gap. In 2022, more than 200,000 migrants crossed the Darien, according to Panama government officials.13
Migrants are vulnerable to various neglected tropical diseases as they traverse this region, including malaria, mosquito-borne arboviruses, and helminth diseases. The congregate settings where migrants shelter also render them vulnerable to communicable diseases such as COVID-19.
As more migrants arrive at our southern border, clinicians will likely encounter migrant patients with fevers. Clinicians must have high suspicion and familiarize themselves with managing tropical diseases. Co-infection of multiple tropical and communicable diseases, as seen in this case, should also be considered.
References
- Ainsley JA. Migrant border crossings in Fiscal Year 2022 topped 2.76 million, breaking previous record. NBCNews.com. October 22, 2022. Accessed November 11, 2023. https://www.nbcnews.com/politics/immigration/migrant-border-crossings-fiscal-year-2022-topped-276-million-breaking-rcna53517.
- Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2(9):e300. Published 2008 Sep 24. doi:10.1371/journal.pntd.0000300
- White NJ, Breman JG. MALARIA. In: Kasper DL, Fauci AS. eds. Harrison's Infectious Diseases, 3e. McGraw Hill; 2017. Accessed November 11, 2023. https://accessbiomedicalscience.mhmedical.com/content.aspx?bookid=2073§ionid=156825301
- Kuhn JH, Charrel RN. Arthropod-Borne and Rodent-Borne Virus Infections. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. eds. Harrison's Principles of Internal Medicine, 20e. McGraw Hill; 2018. Accessed November 11, 2023. https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192026062
- Mace KEE, Lucchi NW, Tran KR. Malaria Surveillance — United States, 2018. Centers for Disease Control and Prevention. 2022. Accessed November 11, 2023. https://www.cdc.gov/mmwr/volumes/71/ss/pdfs/ss7108a1-h.pdf.
- Bardach A, Ciapponi A, Rey-Ares L, et al. Epidemiology of Malaria in Latin America and the Caribbean from 1990 to 2009: Systematic Review and Meta-Analysis. Value Health Reg Issues. 2015;8:69-79. doi:10.1016/j.vhri.2015.05.002
- Valdivia HO, Villena FE, Lizewski SE, Garcia J, Alger J, Bishop DK. Genomic surveillance of Plasmodium falciparum and Plasmodium vivax cases at the University Hospital in Tegucigalpa, Honduras. Sci Rep. 2020;10(1):20975. Published 2020 Dec 1. doi:10.1038/s41598-020-78103-w
- Sinka ME, Bangs MJ, Manguin S, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. Published 2011 May 25. doi:10.1186/1756-3305-4-89
- Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. Published 2015 Jun 30. doi:10.7554/eLife.08347
- Dimaio MA, Pereira IT, George TI, Banaei N. Performance of BinaxNOW for diagnosis of malaria in a U.S. hospital. J Clin Microbiol. 2012;50(9):2877-2880. doi:10.1128/JCM.01013-12
- Central American Refugee Health Profile. Centers for Disease Control and Prevention. November 30, 2021. Accessed November 11, 2023. https://www.cdc.gov/immigrantrefugeehealth/profiles/central-american/index.html.
- Hotez PJ, Damania A, Bottazzi ME. Central Latin America: Two decades of challenges in neglected tropical disease control. PLoS Negl Trop Dis. 2020;14(3):e0007962. Published 2020 Mar 26. doi:10.1371/journal.pntd.0007962
- International Organization for Migration. OFICINA REGIONAL PARA AMÉRICA DEL SUR. February 2023. Accessed November 11, 2023. https://robuenosaires.iom.int/sites/g/files/tmzbdl626/files/documents/2023-04/Tendencias-Migratorias-en-las-Americas-ESP-Feb-2023.pdf.
- Surveillance and control of Aedes aegypti and aedes albopictus in the United States. US Department of Health and Human Services. 2018. Accessed November 11, 2023. https://www.cdc.gov/mosquitoes/pdfs/mosquito-control-508.pdf.
- Historic Data (2010-2022). Centers for Disease Control and Prevention. January 19, 2023. Accessed November 11, 2023. https://www.cdc.gov/dengue/statistics-maps/historic-data.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdengue%2Fstatistics-maps%2F2022.html.
- Dengue virus testing. Quest Diagnostics. Accessed November 11, 2023. https://www.questdiagnostics.com/healthcare-professionals/clinical-education-center/faq/faq169#accordion-049d49775e-item-73fe28b2d6.
- Azanza JR, Mensa J, González Del Castillo J, et al. Interactions listed in the Paxlovid fact sheet, classified according to risks, pharmacological groups, and consequences. Rev Esp Quimioter. 2022;35(4):357-361. doi:10.37201/req/054.2022
- World Malaria Report 2022. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO.



