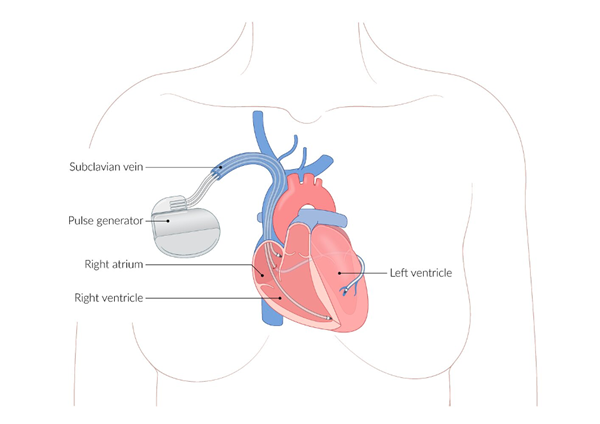
Permanent pacemakers (PPMs) and Implantable Cardioverter-Defibrillators (ICDs) are cardiac implantable electronic devices (CIEDs) that provide pacing support and/or defibrillation in the event of shockable ventricular dysrhythmias.
In the United States, an estimated 300,000 CIEDs are placed annually.1 Given the prevalence of these devices, it is important that emergency clinicians understand their basic operation and are able to recognize and address complications associated with CIEDs. This article reviews the function of PPMs and ICDs, explores the presentation and management of common complications, and briefly discusses newer subcutaneous and leadless device models.
Shared Characteristics Between Devices
Both PPMs and ICDs share a common construction. Transvenous leads with electrical conductance capabilities are advanced through the subclavian vein into the superior vena cava (SVC) and into the right heart, where they are left to detect dysrhythmias, provide electrical impulses to correct dysrhythmias, provide appropriate pacing to the heart, or some combination thereof. These leads are connected to a subcutaneously placed pulse generator that houses a battery, the circuitry responsible for interpreting dysrhythmias and determining appropriate therapy, and capacitors for delivering electrical charge for pacing and/or defibrillation. The pulse generator is typically placed prepectorally and subcutaneously in the left anterior infraclavicular area, although some patients may have a subpectoral placement.
Both PPMs and ICDs can be interrogated using a range of devices provided by the device manufacturer to determine their correct function. If a patient with a PPM or ICD presents to the emergency department with a chief complaint potentially related to cardiac function, make sure to interrogate their device to determine it is functioning properly.
AMBOSS. Cardiac Implantable Electrical Devices (CIEDs). Images used with permission.
PERMANENT PACEMAKER (PPM)
What is it?
A permanent pacemaker (PPM) is a transvenous electronic device that provides pacing support to the heart in patients with bradydysrhythmias in order to maintain sufficient cardiac output and circulation.
Who gets one?
Sinus node dysfunction and high-grade or symptomatic AV block are the most common indications for which patients receive PPM placement.2 Sinus node dysfunction includes symptomatic sinus bradycardia <40 bpm and symptomatic chronotropic incompetence, in which the sinus nodal response to exercise or stress is impaired. Biventricular pacemakers are indicated for patients with left bundle branch block, dilated cardiomyopathy, and congestive heart failure with EF <35% and QRS complex duration >150 msec.2
How does it work?
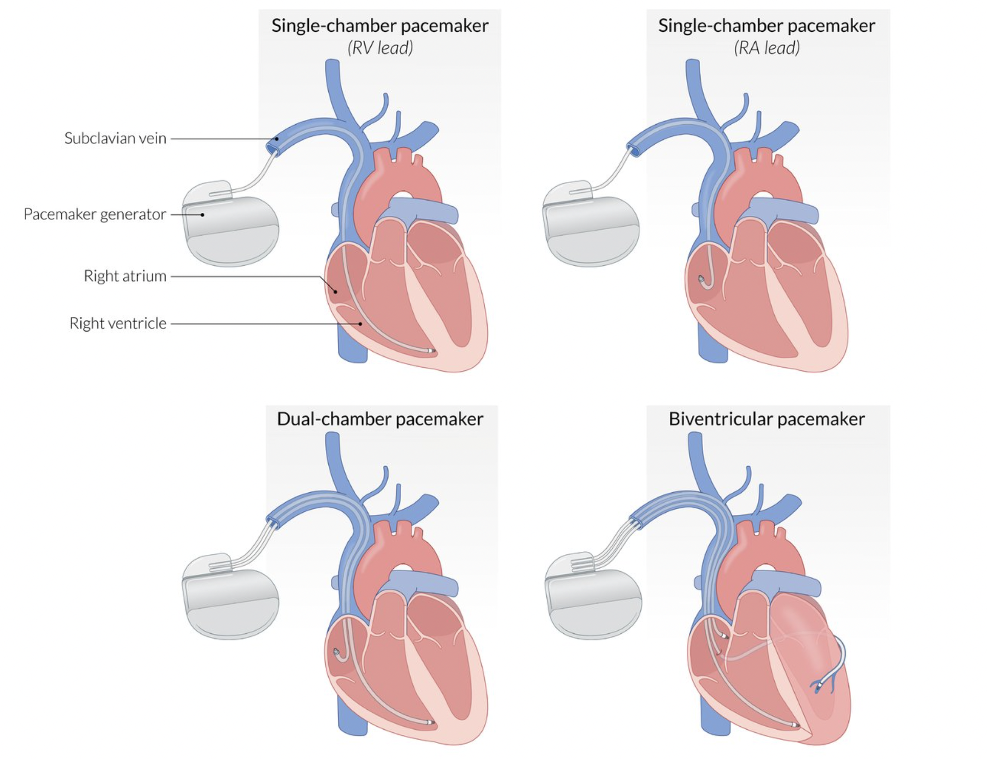
Transvenous leads are advanced to various locations within the right heart depending on the pacemaker model and intended function:
- Single-chamber pacemakers have a single lead that is either placed in the right atrium to stimulate the SA node or in the apex of the right ventricle to provide ventricular pacing.
- Dual-chamber pacemakers have two leads–one for the SA node and another for ventricular pacing.
- Biventricular pacemakers are placed in order to provide cardiac resynchronization therapy (CRT) in which both ventricles are paced to contract in unison. One lead is placed in the RA, another in the RV, and the third is inserted into the coronary sinus to pace the left ventricle.
The ABCs of CIEDs (Pacing Modes)3
|
Position I |
Position II |
Position III |
Position IV |
Position V |
|
Chamber(s) Paced |
Chamber(s) Sensed |
Response to Sensing |
Rate Modulation |
Multisite Pacing |
|
O = None |
O = None |
O = None |
O = None |
O = None |
|
A = Atrium |
A = Atrium |
T = Triggered |
R = Rate Modulation |
A = Atrium |
|
V = Ventricle |
V = Ventricle |
I = Inhibited |
|
V = Ventricle |
|
D = Dual (Atrium and Ventricle) |
D = Dual (Atrium and Ventricle) |
D = Dual (Triggered and Inhibited) |
|
D = Dual (Atrium and Ventricle) |
Position I: Indicates which chambers are paced by PPM leads.
Position II: Indicates which chambers are sensed by PPM leads.
Position III: Indicates how the PPM responds to sensed events:
- T: If the PPM senses an event, it will trigger an output pulse.
- I: If the PPM senses an event, it will inhibit an output pulse for one or more timing cycles.
- D: Only in dual-chamber systems. If an atrial event is sensed, the atrial pulse is inhibited and the ventricular pulse is triggered in timing with the patient’s native PR interval. If a native ventricular event is sensed during the timing delay, the ventricular pulse is inhibited.
Position IV: Indicates whether the PPM will modulate its pacing rate in conjunction with patient activity to maintain adequate cardiac output during exertion.
Position V: Indicates the presence of multiple pacing sites within the respective chambers.
The first 3 positions are generally the most important for emergency physicians confronted with pacemaker-related dysrhythmias or malfunction.
IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR (ICD)
What is it?
An Implantable Cardioverter/Defibrillator (ICD) is a transvenous electronic device designed to sense and correct ventricular tachydysrhythmias (VT) and deliver appropriate electrical defibrillation in the case of ventricular fibrillation (VF). Similar to external defibrillation, ICDs deliver electrical impulses to correct life-threatening VT/VF. More recent ICDs record and store electrocardiogram (ECG) tracings from tachydysrhythmia events, which may be accessed and analyzed remotely by clinicians.
Who gets one?
ICDs are indicated for primary prevention of sudden cardiac death (SCD) in patients who are at increased risk of developing VT/VF despite optimal medical management, for secondary prevention of SCD in patients with prior episodes of sustained VT/VF, or who have been resuscitated after SCD due to suspected VT/VF. This includes patients with Brugada syndrome, congenital long QT syndrome, certain cardiomyopathies with reduced left ventricular ejection fraction (LVEF), and patients with resuscitated VT/VF in which a completely reversible cause (eg, MI <48 hrs prior) cannot be identified.
How does it work?
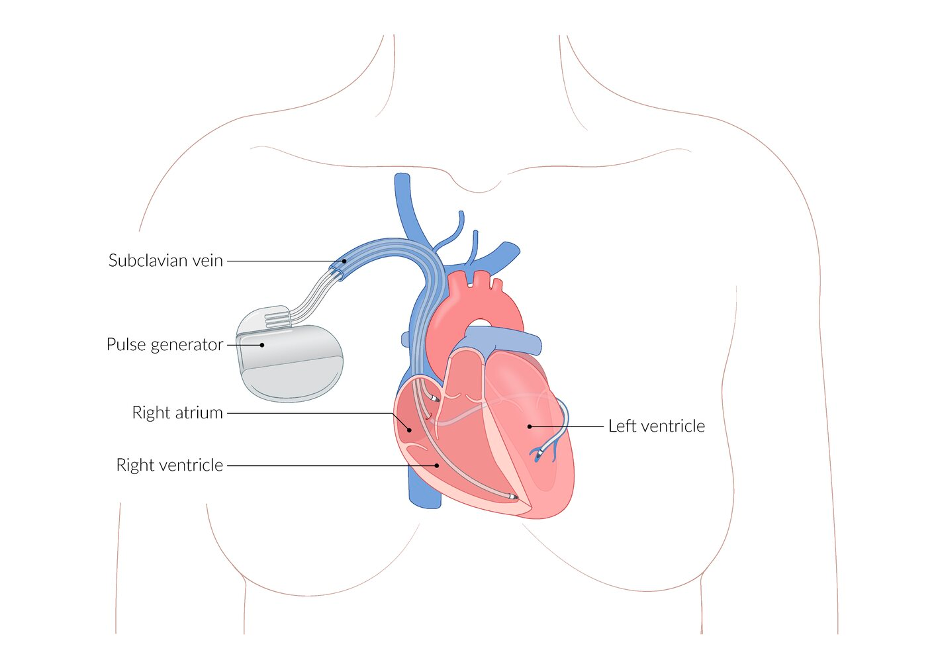
To achieve its purpose of detecting and correcting VT/VF, the ICD is composed of three main elements:
- Sensing/Pacing Electrodes: An electrical lead advanced through the SVC and placed in the RV to detect tachydysrhythmias and deliver antitachycardia “overdrive” pacing when appropriate.
- In overdrive pacing, the electrode delivers a short burst of rapid electrical impulses at a slightly faster rate than the tachycardia; this depolarizes a segment of the reentrant circuit, causing it to enter a refractory period during which it cannot be depolarized, aiming to terminate the tachycardia.
- Defibrillating Electrodes: An electrical coil designed to deliver sufficient electrical current to the heart to defibrillate in the case of VF. Most modern ICDs have a single coil along the ventricular lead (“single coil lead”), while other models may have a second coil proximal to the RV coil that can decrease the necessary defibrillation threshold (“dual coil lead”).
- Pulse Generator: The “body” of the ICD that houses the battery, circuitry for interpreting dysrhythmias and determining appropriate intervention (eg, overdrive pacing, defibrillation), and high-voltage capacitors to build up and store defibrillation potentials.
AMBOSS. Cardiac Implantable Electrical Devices (CIEDs). Images used with permission.
What can go wrong?
Given the related structure, vascular deployment, and function of PPMs and ICDs, they share many of the same complications. The main differences become apparent when these devices malfunction and cannot serve their respective purposes (eg, failure to pace in PPMs, failure to shock VT/VF in ICDs). In general, management decisions should be made in close consultation with a cardiologist.
PERI-PROCEDURAL COMPLICATIONS
Myocardial Perforation
- Definition: Perforation of the myocardium by the device lead acutely during placement, subacute (24 hours - 1 month after placement), or delayed (>1 month after placement).4
- Risk Factors: Older age, female sex, left bundle branch block, worsened heart failure class, higher LVEF, non-single chamber implant.5
- Presentation: May be asymptomatic, but the patient typically has chest pain, dyspnea, or syncope. EKG can show low voltage or electrical alternans and POCUS will show pericardial effusion or signs of cardiac tamponade.4
- Management: If cardiac tamponade, urgent pericardiocentesis and pericardial drain placement. Consult cardiology for appropriate removal and replacement of incorrectly placed lead.
Pneumothorax
- Definition: Intrapleural air accumulation due to perforation of the lung pleura by device components. Increased intrapleural pressure can lead to lung collapse and may progress to tension pneumothorax.
- Risk Factors: Age >80 years, female sex, chronic obstructive pulmonary disorder, non-single chamber implant.6
- Presentation: Pleuritic chest pain, dyspnea, decreased breath sounds. Tension pneumothorax may present with unilateral chest expansion, distended neck veins, tracheal deviation and hemodynamic instability.
- Management: Depending on size and if tension physiology is present, oxygen supplementation and observation versus decompression and urgent tube thoracostomy.
Venous Thrombosis
- Definition: Formation of a blood clot at site of vascular access or anywhere along the length of the transvascular lead.
- Risk Factors: Previous use of temporary transvenous leads, placement of multiple leads, LVEF≤40%7,8
- Presentation: May be clinically silent, but can rarely cause subclavian vein occlusion. Potential for subclavian occlusion may present with upper extremity swelling due to venous congestion.9
- Management: Duplex sonography to determine vascular occlusion.9 Mixed evidence about the role of anticoagulants in upper extremity DVT,10,11 with general recommendations to treat with anticoagulant therapy similar to provoked LE DVT.12
Pocket Infection
- Definition: Local infection of the subcutaneous pocket into which the pulse generator is placed. Commonly due to translocation of epidermal resident flora–Staphylococcus aureus or coagulase-negative strep (eg, epidermidis).13
- Presentation: Pain, swelling, erythema, purulent discharge, erosion or necrosis of the pocket site. Chronic pocket site infection may present only with erosion of the device.
- Management: Early empiric antibiotic treatment. Blood cultures. Consult cardiology for likely removal of the device.
Systemic Infection
- Definition: Infection of the transvenous portion of the lead, serving as a nidus for bacteremia. Commonly Staphylococcus aureus due to contamination during placement.13 Very rarely gram-negative bacteremia (eg, Pseudomonas aeruginosa).14
- Presentation: Fever, chills, can progress to sepsis and septic shock. Cardiac murmurs may be present in case of lead or valve vegetations. Increased risk of venous thrombosis.
- Management: Early empiric systemic antibiotic treatment. Blood cultures. Imaging (eg, TEE) to assess for lead or valve vegetation. Cardiology should be consulted to determine if lead needs to be removed. The device may be retained if the bacteremia is caused by a defined source other than the device and the CIED has not been seeded. Criteria for retention include:15
- Bacteremia (other than aureus) from a clearly defined source other than the CIED, if:
- There is no clinical or imaging (eg, TEE) evidence of lead or valve infection
- There is no evidence of a pocket infection
- The device has not been manipulated within the past 3 months
- Bacteremia (other than aureus) from a clearly defined source other than the CIED, if:
LONG-TERM COMPLICATIONS
The anticipated lifetime for a pacemaker is 7-12 years. Newer pacemakers have a much lower failure and complication rate than legacy devices.
Lead Failure or Malfunction
- Definition: Electrical failure of the leads to function appropriately, resulting in failure to pace, failure to shock, or delivery of inappropriate shocks.
- Presentation: Symptoms related to the failed/malfunctioning lead. May present with tingling sensation in upper extremity due to inappropriate discharge from the pulse generator and/or leads.
- Failure to Pace: Dizziness, syncope, evidence of heart failure (eg, pedal edema, elevated jugular venous pressures) resulting from uncorrected bradycardia.
- Failure to Shock: Uncorrected episode of VT/VF despite device placement due to device malfunction.
- Inappropriate Shock: Chest pain or discomfort due to delivery of inappropriate shocks when no VT/VF actually present. May also result in arrhythmia.
- Management: Device replacement is indicated in instances of malfunction. Manage the patient appropriately for presenting symptoms and consult cardiology for evaluation of device function and consideration for lead extraction.
Lead Dislodgement / Displacement
- Definition: Dislocation of leads from pulse generator or from their position in the heart due to mechanical force such as trauma. Twiddler’s Syndrome is dislodgement of the device due to patient manipulation (ie, twiddling with the device).16
- Presentation: Bradycardia due to faulty pacing, or failure to detect and shock VT/VF.
- Management: Treat presenting tachydysrhythmia or bradydysrhythmia. Chest x-ray to determine location of lead. Cardiology consult for reconnection of device.
Pacemaker Syndrome
- Definition: Atrioventricular asynchrony as a result of suboptimal pacing by CIED, resulting in adverse hemodynamic and electrophysiological consequences.17,18
- Risk Factors: Lower baseline sinus rate, higher programmed ventricular rate, higher percentage of paced beats.19
- Presentation: Dyspnea, orthopnea, cough, chest discomfort, easy fatigability. Exam may reveal signs of decompensated heart failure (crackles, JVD, peripheral edema).18
- Management: Treatment of patient heart failure (diuresis, afterload reduction, noninvasive positive pressure ventilation if needed). Cardiology consult to upgrade pacing mode.19
Pacemaker Mediated Tachycardia (PMT) / Pacemaker-induced Reentry Tachycardia
- Definition: Excitation of ventricles due to detected atrial activity (eg, atrial fibrillation) can cause retrograde conduction through the atrioventricular (AV) node, restimulating the atrium and causing the device to cyclically re-excite the ventricles.20
- Presentation: Palpitations, chest pain, dizziness, syncope. Wide complex tachycardia on EKG. May be asymptomatic.
- Management: Application of magnet to pacemaker pocket will cause it to enter asynchronous pacing mode, preventing response to atrial re-entry. Note that magnet placement on an ICD will also turn off cardioversion/defibrillation. Magnet effects last only while the magnet is on the device. Carotid massage and AV nodal blocking pharmacologic therapy with adenosine, beta-blockers, digoxin, or non-dihydropyridine calcium channel blockers (eg, diltiazem, verapamil).20
Tricuspid Regurgitation (TR)
- Definition: Systolic regurgitation of blood from the RV into the RA due to tricuspid valve insufficiency. Can be caused by mechanical trauma to the valve during lead placement, or mechanical prevention of appropriate valve closure by indwelling lead resulting in regurgitation.
- Presentation: Patients may have decreased exercise tolerance and increased fatigue. Exam may reveal murmur, pitting edema, or other signs of right sided heart failure.
- Management: Imaging (eg, TTE) may help determine structure and functional status of the tricuspid valve. Medical management of symptomatic TR / right heart failure with appropriate diuretics. Cardiology should be consulted to determine if the device should be removed or if retention is feasible with medical optimization.21
Phantom Shocks
- Definition: Perception of having received a shock from an ICD when no such shock was actually delivered, and/or anticipatory anxiety about receiving such shocks.
- Risk Factors: Prior delivery of actual shock. Underlying anxiety disorder.22,23
- Presentation: Anxiety, chest pain, discomfort related to perceived delivery of shock without evidence of device having delivered any such shock (eg, shock delivery recorded in device database).
- Management: Interrogate patient’s device to ensure proper function. Counsel patients, and consider follow-up with outpatient cardiology. Episodes tend to abate over time.
IMPORTANT CONSIDERATIONS
CIED placement is NOT a contraindication for CPR and/or external defibrillation in the case of VT or VF after device failure. If the CIED should return to function and deliver a shock during CPR, the person performing CPR will not be injured by the shock.24 External defibrillation pads should not be placed over or near the device.24
CIED is not an automatic contraindication for imaging with MRI, but caution must be taken before MRI can be performed. Though many modern CIEDs are MRI safe, many CIEDs in the US are legacy devices (ie, placed prior to development of MRI-safe devices), and as such are classified as MRI unsafe.25,26 A radiology safety officer or the device manufacturer should be consulted prior to MRI to ensure safety and prevent magnetic interference with the device. Often even MRI safe devices need to be placed into a special MRI mode by the company technician prior to performing the imaging study. Patients are instructed to carry an identification card with their device safety information on it.
WHAT LIES AHEAD?
Subcutaneous Implantable Cardioverter-Defibrillators (S-ICDs)
Subcutaneous ICDs are similar in construction to transvenous ICDs (TV-ICDs), with a pulse generator connected to a lead for delivering defibrillator shocks. However, unlike TV-ICDs, the lead in S-ICDs is not introduced directly into the heart, but is instead placed subcutaneously over the anterior surface of the heart at the left parasternal margin. S-ICDs may therefore be considered over TV-ICDs in patients who have an increased risk of complications related to intravenous device placement, or in younger patients who have an anticipated need for ICD therapy over several decades (eg, channelopathies).27,28 Importantly, S-ICDs cannot provide pacing support.
Compared to TV-ICDs, S-ICDs have reduced lead-related complications (eg, pneumothorax, myocardial perforation, venous thrombosis); both devices have similar rates for non-lead-related complications such as inappropriate shocks and pocket infections.29
Leadless Permanent Pacemakers
Leadless PPM models are composed of a single unit, containing both the pulse generator and electrode, that is implanted transvenously in the right ventricle. They provide only single chamber pacing. Their development was in response to complications associated with traditional transvenous models, including tricuspid valve injury, venous thrombosis, and pocket infections.30
Pre-approval trials showed reduced rates of major complications compared to transvenous systems.30 The most common complications were device dislodgement, myocardial perforation, and elevated pacing threshold requiring device repositioning.31 Post-marketing studies are ongoing to determine the rates of long-term complications and safety profiles for these devices.
TAKE-HOME POINTS
- Have a low threshold to interrogate a patient’s CIED to determine proper function of the device.
- A CIED is not a contraindication for external defibrillation or CPR. The device will not be damaged by the delivery of electricity.
- An internal shock delivered by the CIED will not harm a person performing CPR.
- Though many devices are MRI safe, consultation with device manufacturer or radiology safety officer is necessary before scanning.
- CIEDs can be quickly switched into asynchronous pacing mode through placement of a magnet over the patient’s chest.
References
- Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540-1545.
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(7):e51-e156.
- Bernstein AD, Daubert JC, Fletcher RD, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol PACE. 2002;25(2):260-264.
- Migliore F, Zorzi A, Bertaglia E, et al. Incidence, Management, and Prevention of Right Ventricular Perforation by Pacemaker and Implantable Cardioverter Defibrillator Leads. Pacing Clin Electrophysiol. 2014;37(12):1602-1609.
- Hsu JC, Varosy PD, Bao H, Dewland TA, Curtis JP, Marcus GM. Cardiac perforation from implantable cardioverter-defibrillator lead placement: insights from the national cardiovascular data registry. Circ Cardiovasc Qual Outcomes. 2013;6(5):582-590.
- Ogunbayo GO, Charnigo R, Darrat Y, et al. Incidence, predictors, and outcomes associated with pneumothorax during cardiac electronic device implantation: A 16-year review in over 3.7 million patients. Heart Rhythm. 2017;14(12):1764-1770.
- Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2007;9(5):328-332.
- Da Costa SS do C, Scalabrini Neto A, Costa R, Caldas JG, Martinelli Filho M. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol PACE. 2002;25(9):1301-1306.
- Zuber M, Huber P, Fricker U, Buser P, Jäger K. Assessment of the subclavian vein in patients with transvenous pacemaker leads. Pacing Clin Electrophysiol PACE. 1998;21(12):2621-2630.
- Rajasekhar A, Streiff MB. How I treat central venous access device-related upper extremity deep vein thrombosis. Blood. 2017;129(20):2727-2736.
- Debourdeau P, Kassab Chahmi D, Le Gal G, et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol Off J Eur Soc Med Oncol. 2009;20(9):1459-1471.
- Kucher N. Deep-Vein Thrombosis of the Upper Extremities. N Engl J Med. 2011;364(9):861-869.
- Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of Cardiac Implantable Electronic Device Infections. JACC Clin Electrophysiol. 2016;2(4):498-505.
- Pascale R, Toschi A, Aslan AT, et al. Risk factors for Gram-negative bacterial infection of cardiovascular implantable electronic devices: multicentre observational study (CarDINe Study). Int J Antimicrob Agents. 2023;61(3):106734.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement from the American Heart Association. Circulation. 2010;121(3):458-477.
- Weir RAP, Murphy CA, O’Rourke B, Petrie CJ. Twiddler’s syndrome: a rare cause of implantable cardioverter defibrillator malfunction. Eur Heart J. 2016;37(46):3439.
- Ausubel K, Furman S. The pacemaker syndrome. Ann Intern Med. 1985;103(3):420-429.
- Prinzen FW, Strik M, Regoli F, Auricchio A. 9 - Basic Physiology and Hemodynamics of Cardiac Pacing. In: Ellenbogen KA, Kay GN, Lau CP, Wilkoff BL, eds. Clinical Cardiac Pacing, Defibrillation and Resynchronization Therapy (Fourth Edition). W.B. Saunders; 2011:203-233.
- Link MS, Hellkamp AS, Estes NAM, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular-based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43(11):2066-2071.
- Abu-haniyeh A, Hajouli S. Pacemaker Mediated Tachycardia. In: StatPearls. StatPearls Publishing; 2022. Accessed March 18, 2023.
- Addetia K, Harb SC, Hahn RT, Kapadia S, Lang RM. Cardiac Implantable Electronic Device Lead-Induced Tricuspid Regurgitation. JACC Cardiovasc Imaging. 2019;12(4):622-636.
- Prudente LA, Reigle J, Bourguignon C, Haines DE, DiMarco JP. Psychological indices and phantom shocks in patients with ICD. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2006;15(3):185-190.
- Jacob S, Panaich SS, Zalawadiya SK, et al. Phantom shocks unmasked: clinical data and proposed mechanism of memory reactivation of past traumatic shocks in patients with implantable cardioverter defibrillators. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2012;34(2):205-213.
- Pitcher D, Soar J, Hogg K, et al. Cardiovascular implanted electronic devices in people towards the end of life, during cardiopulmonary resuscitation and after death: guidance from the Resuscitation Council (UK), British Cardiovascular Society and National Council for Palliative Care. Heart. 2016;102(Suppl 7):A1-A17.
- Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116(24):2878-2891.
- American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2614-2662.
- Huang J, Patton KK, Prutkin JM. Concomitant Use of the Subcutaneous Implantable Cardioverter Defibrillator and a Permanent Pacemaker. Pacing Clin Electrophysiol PACE. 2016;39(11):1240-1245.
- Aziz S, Leon AR, El-Chami MF. The subcutaneous defibrillator: a review of the literature. J Am Coll Cardiol. 2014;63(15):1473-1479.
- Basu-Ray I, Liu J, Jia X, et al. Subcutaneous Versus Transvenous Implantable Defibrillator Therapy: A Meta-Analysis of Case-Control Studies. JACC Clin Electrophysiol. 2017;3(13):1475-1483.
- Duray GZ, Ritter P, El-Chami M, et al. Long-term performance of a transcatheter pacing system: 12-Month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14(5):702-709.
- Sperzel J, Defaye P, Delnoy PP, et al. Primary safety results from the LEADLESS Observational Study. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2018;20(9):1491-1497.