External ventricular drainage (EVD), is one of the most frequently performed neurosurgical procedures, with approximately 20,000 performed annually.1 EVD placement is often considered a lifesaving measure to alleviate severe hydrocephalus or elevated intracranial pressure (ICP).1
A major benefit of EVD placement is the ability to monitor ICP and also remove fluid with the same device. Current guidelines recommend a staged approach to management of elevated intracranial pressure, with escalation when more conservative efforts are unsuccessful at managing elevated pressures.2 The purpose of this review will be to discuss the devices themselves in regard to their clinical management and troubleshooting. For a more in-depth review of the general management of ICP check out EMRA’s ICP Deep Dive.
As previously mentioned, an EVD is placed mainly to alleviate acute hydrocephalus or elevated ICP. This may be due to a myriad of etiologies, such as SAH, intraparenchymal hemorrhage (with or without ventricular extension), infections, tumors, existing shunt failure, etc.3 While the literature varies in its recommendations and risks associated with the settings of placement, an EVD may be placed in the OR, in the ICU, or in the ED, depending on the needs and stability of the patient.4,5,6
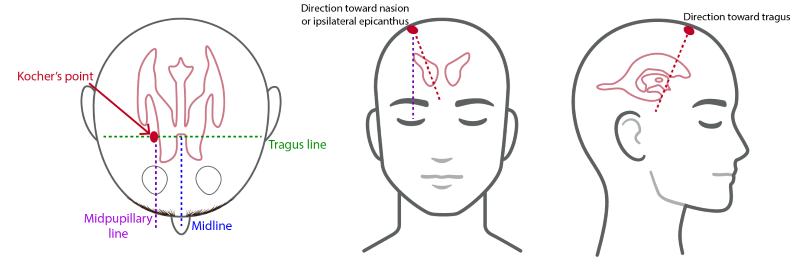
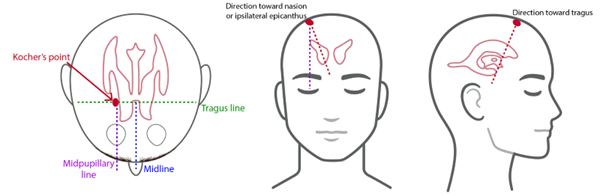
Image 1. Standard anatomic approach and insertion of EVDs7
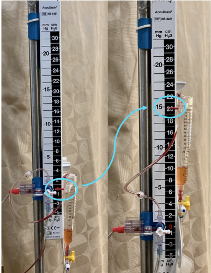
Image 2. Illustrates a standard EVD at a setting of zero (left) and 20 (right) cmH20
The device itself is a drainage catheter placed through the skull with the goal of the distal tip of the catheter resting in the ipsilateral lateral ventricle ideally in the Foramen of Monro. This is achieved by landmark identification most commonly using Kocher's point, while other less common approaches such as Frazier’s point are occasionally used as well (Image 1).
As can be seen in Image 2, the catheter exiting the brain is then connected to a drainage system, which allows for continuous or intermittent draining of intraventricular contents. On the left side of the image you will see an EVD set to 0 cmH2O. It is imperative that this marker aligns with the patient’s tragus. Every EVD will also have a collection chamber, seen here on the right side of the device partially filled with fluid drained from the ventricles. The height of this chamber is what sets the gradient for drainage from the EVD.
The drainage observed is dependent upon the difference between the ICP and the set height of the chamber. For example if you set the chamber to 5 cmH2O you will encourage drainage as standard ICP is often greater than 5. If the chamber is set to 20 cmH2O you will not as readily observe drainage. It is important to acknowledge that many EVD systems have markings represented in cmH2O while the guidelines regarding standard and abnormal ICP are often represented in mmHg. Some devices such as those seen in Image 2 have those two values displayed side by side to ease interpretation but special care must be taken to recognize if your specific device is displaying values only in cmH2O as these two do not have a 1:1 conversion. For example, a setting of 20 cmH2O equates to an ICP of approximately 15 mmHg. At this setting you will not observe the level of drainage you would with the chamber set to 5 cmH2O. While 15 mmHg is still within the normal range of ICP (7-15 mmHg), you will often see this setting to serve as a release mechanism should the ICP rise above this upper limit of normal drainage into the chamber will ensue to avoid continued increase in ICP. The initial setting will vary based on the etiology responsible for elevated ICP. For example, in the setting of an unsecured aneurysmal SAH the initial setting may be relatively high to avoid any rapid change in transmural pressure across the aneurysm wall in order to decrease risk of rebleeding.8 Comparatively, in patients with massive intraventricular hemorrhage the initial setting will often be lower to encourage drainage to alleviate elevated ICP.9
You must remember never to adjust the height or the head of the bed without first clamping the EVD or you will change the drainage gradient and can risk large volume CSF removal which could be catastrophic. It remains best practice to keep the head of the bed at 30° unless otherwise discussed with neurosurgery.
One of the major benefits of an EVD is they allow drainage of CSF as mentioned but also allow you to transduce an ICP, which enables you to more accurately assess CPP on critically ill patients. In most models of EVD you are not able to drain CSF and transduce an ICP at the same time. For this reason, you must clamp the EVD prior to recording an accurate ICP. This may be confusing as even with the EVD unclamped you will still see a reading on the monitor, but this is inaccurate.
Troubleshooting
If the catheter is set to drain but no fluid is being removed, a few measures may be taken to assess the catheter. These should always be done at minimum with approval of neurosurgery but preferably with the neurosurgeon at bedside as well.
- Step 1: Make sure there are no kinks in the catheter and inspect the insertion site to see if any present sutures have caused obstruction
- Step 2: Check the catheters patency by lowering the drain to 0 you should start to see the drainage of a few drops of CSF. If you don’t this indicates that you are dealing with a low ICP problem and not a device issue. Counterintuitively if this occurs you can raise the EVD to slowly re-expand the ventricles and then restart with a slower drainage strategy.
- Step 3: If steps 1 and 2 do not solve your issue, the next step involves flushing the catheter. This MUST be done with neurosurgery at bedside. This involves, under full sterile technique, flushing a small amount of sterile saline away from the patient and then a small volume toward the patient to remove any blockage. If done incorrectly this can lead to rapid shifts in ventricular volume, tract hemorrhage or infection.
Weaning
In general, a weaning trial should not be initiated until there is clinical improvement in the patient and their underlying pathology. The main conversation regarding EVD weaning centers around the risks and benefits of a rapid versus gradual weaning strategy. Based on neuroimaging and clinical status, the intensivist in conjunction with neurosurgery will decide to take a rapid or gradual weaning approach. In a rapid approach there is closure of the drainage system and the catheter is used for monitoring purposes only. The patient is then monitored for any malignant hydrocephalus which can be rapidly diagnosed and addressed and if none occurs the EVD will be removed.10 The proposed underlying pathophysiology behind a rapid wean strategy is that even if there is an increase in CSF volume following rapid clamping this will lead to the increased recruitment of CSF reabsorption pathways in the arachnoid granulation leading to an appropriate equilibrium between production and reabsorption.11 In a more gradual wean strategy the drain is typically left open and slowly raised over a period of days until the level of the drain is at 25 cmH2O at which point the drain will be clamped and then following no change in clinical status discontinued. The theory behind a gradual wean strategy is that it allows a slower rebalancing between the CSF production and reabsorption pathways.12 There is some variation in the existing literature as to which weaning strategy is superior. It should be noted that most of the available literature is in studies of patients with SAH. Recent studies have shown that early clamping and intermittent drainage is associated with decreased EVD malfunction and length of stay.13,14 More recently a multicenter prospective randomized trial found an early wean strategy was associated with decreased ICU length of stay, decreased VP shunt placement and decreased EVD associated complications.15 Even in the face of the wealth of evidence supporting the benefit of an early wean strategy, a more gradual wean strategy is still employed in a majority of ICUs across the country. Regardless, it should be a multidisciplinary approach involving the intensivist and consulting service who placed the drain.
Intraparenchymal Monitors
There are three main types of intraparenchymal monitors: piezoelectric strain gauge, fiberoptic, and pneumatic.16
- Fiberoptic devices, such as the Camino® ICP monitor, transmit light via a fiberoptic cable toward a displaceable mirror at the catheter tip. The mirror will move in response to changes in ICP, which will then change the intensity of the reflected light and be interpreted as an ICP value.17

Image 3. Camino® Intracranial monitor
- Piezoelectric strain gauze devices, such as the Codman ® microsensor, the Pressio® sensor, and the Raumedic® Neurovent-P ICP sensor, have a transducer that detects pressure-generated changes in electrical resistance and uses that to calculate an ICP.17

Image 4. Codman® Microsensor
- Pneumatic sensors, such as Spiegelberg®, use a small balloon in the distal end of the catheter to register changes in pressure.17
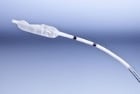
Image 5. Spiegelberg® intracranial probe
Intraparenchymal monitors can be inserted through a skull bolt or tunneled under the skin from a burr hole.18 They are usually placed in the right frontal region at a depth of approximately 2 cm, though other sites can also be selected depending on the regional pathology in the brain.
Compared to EVDs, the advantages of intraparenchymal monitors include simple use and placement, especially in the setting of midline-shift or ventricular effacement, lower risk of infection and bleeding, continuous data feedback, and reliable feedback regardless of patient positioning.19 The main disadvantages include the inability to drain CSF and the fact that once the catheter is placed, it cannot be recalibrated again. Over time, this can result in imprecise ICP values. The difference between the starting ICP value when the sensor is calibrated (0 mmHg) and the ICP value that is measured when the sensor is removed is called “zero drift.” A large zero drift suggests that the ICP measured while the device was in the patient may not be accurate. In addition, the measured ICP from an intraparenchymal monitor may not represent global pressure if there is an intraparenchymal pressure gradient between different regions of the brain, which has been reported in the setting of TBI.18
Sensors can also be placed in the subarachnoid, subdural, and epidural spaces using the pressure-detection technologies mentioned above. While they offer advantages such as being less invasive and more easily placed, these locations are not as commonly used as ventricle and intraparenchymal areas due to concerns for accuracy and reliability.20
ICP Waveform
The ICP waveform is dynamic with respiratory and cardiac cycle correlates. Respiratory variation is between 2 and 10 mmHg due to changes in intrathoracic pressure; however, this variation is obscured in the setting of elevated ICP.
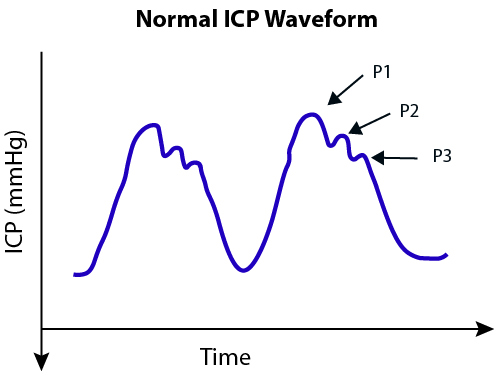
Image 6. Normal intracranial pressure waveform21
- P1 (percussion wave) correlates with arterial pulsation being transmitted from the choroid plexus.
- P2 (tidal wave) is a proxy for intracranial compliance.
- P3 (dicrotic wave) correlates with aortic valve closure.
As brain compliance decreases, the amplitude of P2 will rise to eventually become larger than that of P1.
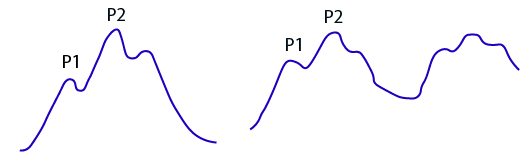
Image 7. Intracranial pressure waveform in the setting of elected ICP21
When ICP measurements are plotted over time, more advanced analysis can be done to identify pathologic waveforms.
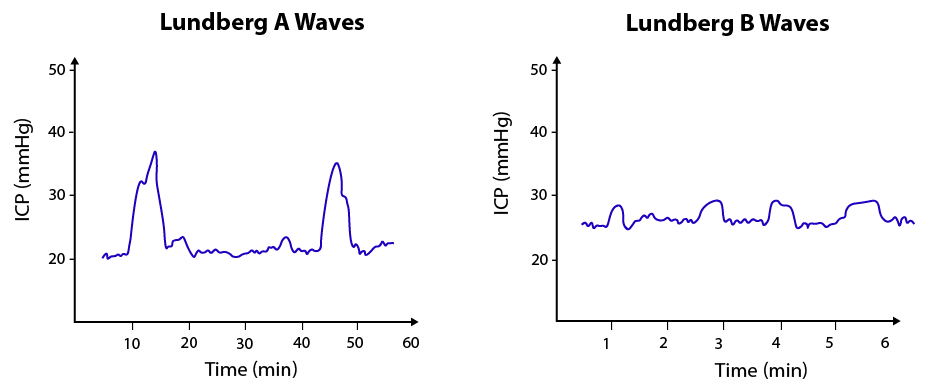
Image 8. Lungberg A and B-waves21
Lundberg A-waves are characterized by rapid increase and decrease of pressures with large amplitude changes lasting from 5-20 minutes. This signals the exhaustion of the brain’s compliance. Lungberg B-waves are sharp with a saw-tooth pattern, occurring every 1 ½ to 2 minutes with a smaller amplitude, and may be a sign of decreased brain autoregulation but can also just be physiologic.
Take-Home Points
- External ventricular drains are devices that are placed in patients with elevated ICP who require both monitoring of the ICP as well as the ability to drain fluid responsible for the ICP elevation.
- EVDs are placed by neurosurgery typically lying in the foramen of Monro and have the benefit of providing actionable information about the ICP of the patient in a continuous manner while also having the capability of draining fluid that is causing an elevation in ICP such as blood or CSF.
- The level at which your EVD is set will dictate at which ICP you will start to drain fluid, and this can be titrated up or down to achieve clinical improvement as well as wean the patient off of the EVD.
- Intraparenchymal ICP monitors on the other hand have the benefit of being less invasive, less risk of infection and bleeding, and they provide continuous feedback and are unaffected by patient positioning. With that being said, they lack the ability to drain fluid and also cannot be recalibrated once placed, two benefits that are enjoyed by EVDs.
- The ICP triphasic waveform provides valuable information about the pressure the tissues in your brain are experiencing, with a prominent P2 indicating a decrease in compliance of the brain.
- When plotted over longer periods of time ICP measurements may provide further information as to the pressures experienced by the brain tissue. These come in the form of Lundberg waves and may further support what is seen on the monitor from pressures measured at a single point in time.
- It is incumbent upon the emergency physician to know the indications for EVD or ICP placement so they may better dictate their patients care and disposition when discussing with subspecialists. Furthermore, the intensivist will undoubtedly encounter these devices and must be comfortable with their management and troubleshooting.
References
- Sekula RF, Cohen DB, Patek PM, Jannetta PJ, Oh MY. Epidemiology of ventriculostomy in the United States from 1997 to 2001. Br J Neurosurg. 2008;22:213-218.
- Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage: Historical vignette. J. Neurosurg. 2014;120(1):228-236.
- Cinibulak Z, Aschoff A, Apedjinou A, Kaminsky J, Trost HA, Krauss JK. Current practice of external ventricular drainage: A survey among neurosurgical departments in Germany. Acta Neurochir. 2016;158:847-853.
- Altschul D, Hamad MK, Kobets A, et al. A Retrospective Quality Analysis of External Ventricular Drain Infection Rates Following Stroke Diagnoses and Other Brain Injuries: Comparison of Emergency Room and ICU/OR Setting. Cureus. 2020;12:e7173.
- Dawod G, Henkel N, Karim N, et al. Does the Setting of External Ventricular Drain Placement Affect Morbidity? A Systematic Literature Review Comparing Intensive Care Unit versus Operating Room Procedures. World Neurosurg. 2020;140:131–141.
- Kohli G, Singh R, Herschman Y, Mammis A. Infection Incidence Associated with External Ventriculostomy Placement: A Comparison of Outcomes in the Emergency Department, Intensive Care Unit, and Operating Room. World Neurosurg. 2018;110: e135–e140.
- Bakhsheshian J, Pellegrin K, Gruen JP. Intracranial Pressure Monitoring and Ventricular Drainage. In: Demetriades D, Inaba K, Lumb P (eds). Atlas of Critical Care Procedures. Springer, Cham. 2018.
- Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012. 12: 24-33
- Hunn BH, Mujic A, Sher I, Dubey AK, Peters-Willke J, Hunn AW. Successful treatment of negative pressure hydrocephalus using timely titrated external ventricular drainage: A case series. Clin Neurol Neurosurg. 2014. 116: 67-71.
- Bertuccio A, Marasco S, Longhitano Y, et al. External Ventricular Drainage: A Practical Guide for Neuro-Anesthesiologists. Clin Pract. 2023;13:219-229.
- Leeds ES, Kong AK, Wise BL. Alternative pathways for drainage of cerebrospinal fluid in the canine brain. Lymphology. 1989;22(3):144-146.
- Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2016;80:6-15.
- Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, Graffagnino C. Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. J Neurosurg. 2013;119(4):974-80.
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, Hott JS, Goslar P, Zabramski JM, Spetzler RF. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg. 2004;100(2):225-9.
- Chung DY, Thompson BB, Kumar MA, et al. Association of External Ventricular Drain Wean Strategy with Shunt Placement and Length of Stay in Subarachnoid Hemorrhage: A Prospective Multicenter Study. Neurocrit Care. 2022;36(2):536-545.
- Anania P, Battaglini D, Pelosi P, Robba C. Type of ICP monitor. In: Prabhakar H, ed. Essentials of Evidence-Based Practice of Neuroanesthesia and Neurocritical Care: Academic Press; 2022.
- Raboel P, Bartek Jr. J, Andresen M, Bellander, Romner B. Intracranial Pressure Monitoring: Invasive versus Non-Invasive Methods—A Review. Critical Care Res Pract 2012; 2012.
- Papadakis S, Ampadiotaki M-M, Pallis D, et al. Cervical Spinal Epidural Abscess: Diagnosis, Treatment, and Outcomes: A Case Series and a Literature Review. J Clin Med. 2023; 12(13):4509.
- Pain M, Francoeur C, Dangayach N, Gordon E, Mayer S. Invasive Multimodality Brain Monitoring. In: Loftus C, ed. Neurosurgical Emergencies. Thieme/AANS; 2018.
- Abraham M, Singhal V. Intracranial pressure monitoring. J Neuroanaesth Crit Care. 2015;2(3):193-203.
- Nag D, Sahu S, Swain A, Kant S. Intracranial pressure monitoring: Gold standard and recent innovations. World J Clin Cases. 2019; 7(13):1535-1553.