A recent paper is calling for significant changes to the emergency treatment of anaphylaxis. This review takes a deep dive into the evidence.
Article
Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ. Evidence update for the treatment of anaphylaxis. Resuscitation. 2021;163:86-96.
Anaphylaxis is a severe life-threatening acute systemic type 1 hypersensitivity reaction. This IgE-mediated reaction leads to varying degrees of mast-cell degranulation, histamine release, and varying levels of symptom severity. Commonly affected organ systems include the respiratory, ocular, cutaneous, cardiovascular, and gastrointestinal systems. Thus, anaphylaxis can present as a spectrum: ranging from mild breathing difficulty, such as wheezing to circulatory compromise and shock.1, 2 The three most common triggers include medications (35%), food (32%), and insect stings (19%).2 In the United States, the prevalence of anaphylaxis in the general population is estimated between 1.6% and 5.1%, with an approximate 0.3% fatality rate.2 Treatment of anaphylaxis leads to direct costs of $1.2 billion and indirect costs of $609 million annually.3, 4
Traditionally, the acute management of anaphylaxis in the emergency department include:1, 5-7
- Evaluation and stabilization of airway, breathing, and circulation
- Intramuscular (IM) injections of epinephrine (0.3 – 0.5 mg) every 5 to 15 min
- Intravenous (IV) crystalloid 1 – 2 L/hr for hypotension
- Nebulized beta-agonists (albuterol 2.5 mg)
- H1 antagonists 25-50 mg IM/IV
- H2 antagonist 20 mg IV
- Corticosteroid 1.0-2.0 mg/kg IV
An article recently published in Resuscitation by Dodd et al.6 in conjunction with The Resuscitation Council UK titled "Evidence update for the treatment of anaphylaxis" recommended significant changes to the emergency treatment of anaphylaxis. In particular, these guidelines recommend against the use of H1/H2 blockers and corticosteroids in an acute setting.6 (see Table 1)
Table 1. Traditional Management V.S. New recommendation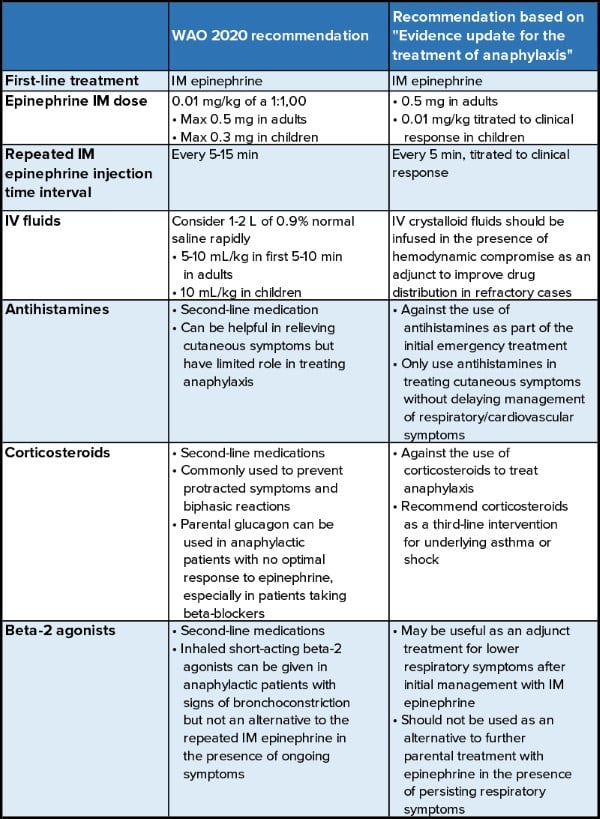
I: Epinephrine
Effectiveness
The use of epinephrine as the first-line medication for the treatment of anaphylaxis is well established and agreed upon among international guidelines.7-10 However, supporting evidence remains limited to observational studies. Prompt epinephrine administration has been demonstrated to have better clinical outcomes when compared to antihistamines and corticosteroids alone.9 A high risk of bias was identified in evaluating the efficacy of epinephrine, as limited randomized controlled trials exist given its established effectiveness.11
Approximately 10% of patients experiencing anaphylaxis show a suboptimal response to one dose of epinephrine, but only 2.2% fail to respond to two doses of epinephrine.12 Furthermore, delayed administration of epinephrine can lead to more severe outcomes such as prolonged reactions, hypotension, and increased mortality.11-13
Though there is a lack of randomized controlled studies comparing critical outcomes with epinephrine intervention versus no epinephrine intervention, epinephrine is suggested to be the most appropriate treatment to reduce morbidity based on existing guidelines. As a result, Dodd et al. recommend the use of epinephrine as the first-line treatment for anaphylaxis.6
Optimal timing of administration
Currently, there is limited evidence comparing the effect of early versus delayed epinephrine administration on clinical outcomes. However, early epinephrine treatment is suggested to be associated with improved outcomes in an out-of-hospital setting,3 whereas delayed administration of epinephrine was found to be associated with a higher rate of biphasic reaction (OR 3.39; 95% CI 1.13-10.18).14 Although data from a meta-analysis15 and European Anaphylaxis Registry16 suggest a limited impact of epinephrine on the occurrence of biphasic reactions (OR 0.91; 95% CI 0.6-1.4 and OR 0.91; 95% CI 0.71-1.16, respectively), the 2020 Joint Task Force on Practice Parameters (JTFPP)8 acknowledged a trend of lower rates of biphasic reactions associated with early administration of epinephrine in anaphylaxis patients. Prophylactic epinephrine use in mild or non-anaphylactic cases has not been shown to prevent progression to anaphylaxis.17 Although there is limited evidence on the optimal timing of epinephrine administration, Dodd et al. recommend epinephrine administration as soon as anaphylaxis is recognized or suspected based on the current consensus reflected among international guidelines.6
Optimal route of administration
Currently, there are no trials assessing different routes of epinephrine administration in anaphylactic patients. However, the traditional IM route has an excellent safety profile and is recommended in all settings according to international guidelines.1 Additionally, IM injection has been shown to be relatively easy and safe in both clinical and non-clinical settings. The subcutaneous route is not recommended, as it has been shown to have lower plasma drug levels than the IM route and carries a higher risk of confounding factors such as depth of injection and different injection sites.11, 18 Epinephrine overdose is defined as one dose that exceeds the dosage recommendation in anaphylaxis guidelines (IM: 0.01 mg/kg with max 0.5 mg; IV bolus: 100 μg).1, 10, 19 Cardiovascular complications such as tachyarrhythmias, severe hypertension, cardiac ischemia, and stroke may arise as a result of epinephrine overdose.6, 19 In comparison to the IM route, IV bolus administration has been shown to have a 13% increase in the incidence of epinephrine overdose and an 8% increase in the incidence of cardiovascular complications.19 As a result, Dodd et al. recommend IM route for initial epinephrine treatment of anaphylaxis.6
Optimal dose of IM administration
The safety and efficacy of IM epinephrine is well established and shown in Table 2.6 A general guideline for the pediatric population is a dose of 0.01 mg/kg (max 500 µg) titrated to clinical response. Higher doses (150µg vs. 300 µg in children, 300 µg vs. 500 µg in adults) showed better absorption profile while the effect on clinical response is unknown.20, 21 Although fewer administration errors were found in using auto-injector (AAI), most AAIs deliver a maximum of 300 µg and may result in underdosing.22 Overall, Dodd et al. recommend administration of 0.5 mg IM in adults, which has been consistently used globally for many decades.6
Table 2. Recommended IM Epinephrine Doses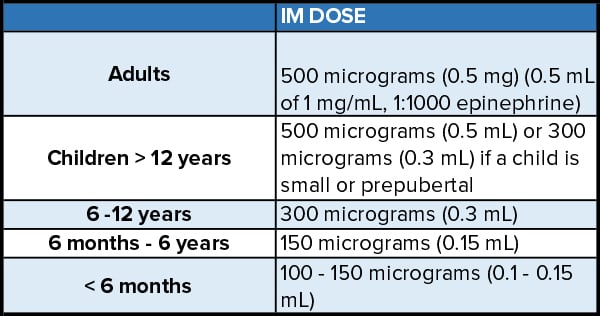
Additional doses in refractory cases
Although many patients respond well to one dose of epinephrine, some may require additional doses. Approximately 10% of patients with anaphylaxis respond suboptimally to a single dose of IM epinephrine, but 98% will respond to 1-2 additional doses.12 Additionally, in a recent observational study by Turner et al., a single dose of IM epinephrine demonstrated limited effect on reversing the cardiovascular effects in peanut-induced anaphylaxis.23 One dose of IM epinephrine resulted in a mean increase of 9.4% on stroke volume (p=.029) compared to the 5-fold greater increase (p=.04) from IV fluids administration.23, 24
Refractory cases can be defined as persistent symptoms of anaphylaxis that require ongoing treatment despite appropriate epinephrine administration and symptomatic treatment.6, 25 Refractory anaphylaxis is likely due to insufficient epinephrine delivery in combination with both inadequate dosing and insufficient circulatory capacity.25, 26 International guidelines suggest repeating doses of epinephrine every 5 – 15 minutes for persistent reactions. However, there is no clear justification for waiting longer than 5 minutes. Low dose epinephrine infusion is included in the Australian and Spanish guidelines as the treatment of choice in refractory cases of severe anaphylaxis.7, 27 Although it is not included in all guidelines, the appropriate use of low dose IV epinephrine infusion is shown to be effective and safe.26, 28 In addition, no clear superiority of second-line vasopressors (dopamine, dobutamine, norepinephrine, phenylephrine, vasopressin) have been demonstrated and their use is suggested only if IV epinephrine is ineffective.7 Adverse effects of epinephrine administration most often occur when given intravenously in cases of overly rapid infusion, bolus, and dosing errors.3, 9 Thus, Dodd et al. recommend the following options in treating refractory anaphylaxis: 1) IM epinephrine every 5 minutes, 2) IV epinephrine infusion with expert support when respiratory/cardiovascular symptoms of anaphylaxis persist after 2 appropriate doses (IM or IV), 3) low dose IV epinephrine infusion due to its favorable effectiveness and safety profile.6
II: Antihistamine
Currently, there is no randomized control trial evidence to support using antihistamines in managing anaphylaxis.1, 3 Oral H1-blockers can treat cutaneous symptoms of anaphylaxis, and it may be more effective when given with H2-blockers rather than alone.3 However, cutaneous symptoms are generally not fatal and respond well to epinephrine. Treatment with antihistamines does not show to improve survival and it has no role in treating respiratory/cardiovascular symptoms of anaphylaxis.8, 29, 30 Possible adverse effects of H1 blockers include sedation (which can confound anaphylactic symptoms) and possible hypotension (if infused via IV bolus rapidly).1, 3, 7, 31 In addition, treatment with antihistamines may result in delayed epinephrine administration.1, 3, 7, 8 Prehospital use of antihistamines was associated with delayed presentation to healthcare facilities, a lower rate of >1 dose epinephrine administration, and increased morbidity.32, 33 Antihistamines are associated with an increased occurrence of biphasic reactions rather than a reduction.8, 15, 16 Therefore, Dodd et al.6 recommend: 1) not using antihistamines as part of the initial treatment, and 2) only use antihistamines to treat cutaneous symptoms after appropriate management of respiratory and cardiovascular symptoms of anaphylaxis.
III: Corticosteroid
Due to the slow-acting nature and their mechanism of action, corticosteroids are likely to have limited therapeutic effect in an acute setting of anaphylaxis. Thus, the use of corticosteroids is primarily for the prevention of biphasic reactions.8, 32 However, there is a lack of strong evidence to support whether the use of corticosteroids results in changes in reaction severity or the occurrence of biphasic reactions.8, 34 Same as antihistamines, corticosteroids are found to be given more frequently than epinephrine in acute anaphylaxis and this may result in delayed epinephrine administration.32, 33, 35-41 Emerging evidence indicates that routine use of corticosteroids in anaphylaxis may be associated with increased morbidity.32, 42 Hospitalization and admission to the ICU are also found to be associated with prehospital treatment with corticosteroids (adjusted OR 2.84) after adjusting for symptom severity and treatments administered.32 Thus, Dodd et al.6 recommend 1) against the routine use of corticosteroids to treat anaphylaxis, and 2) only use corticosteroids as a third-line intervention to treat underlying asthma/shock.
IV: IV fluids
IV fluids are beneficial in anaphylactic patients with cardiovascular instability. IV fluid infusion can restore circulatory volume and may enhance epinephrine delivery and faster symptom resolution.1, 3, 7 In addition, crystalloid fluid 500-1000 mL was shown to have greater effect in restoring venous return in comparison to one dose of IM epinephrine.24 Thus, Dodd et al.6 recommend:
- IV crystalloid fluids infusion in the presence of hemodynamically compromised
- IV crystalloid bolus as an adjunct in refractory cases.
V: Inhaled beta-agonists
There is limited evidence to support the use of inhaled beta-2 agonists/bronchodilators in treating anaphylaxis in an acute setting.1, 3, 9 Beta-2 agonists may be beneficial for persistent wheeze, but they do not reduce or prevent upper airway obstructions and hypotension.1, 3, 7, 43 Evidence shows that in anaphylaxis cases that were initially misdiagnosed as severe asthma, patients did not respond to parental bronchodilators but did respond to epinephrine.44, 45 Therefore, parental bronchodilators should not be used to replace epinephrine in managing acute anaphylaxis. Dodd et al.6 recommend: 1) using beta-2 agonists as an adjunct treatment for lower respiratory symptoms after initial IM epinephrine administration, and 2) not to be used as an alternative to further parental epinephrine treatment in the presence of persisting respiratory symptoms.
Summary
The Resuscitation Council UK recently updated its guideline on the acute management of anaphylaxis. In this article, we reviewed and discussed the traditional management options and the proposed new changes. Adapted from international consensus, epinephrine remains the first-line treatment of choice for anaphylaxis.
- The optimal timing for epinephrine administration is once symptoms of anaphylaxis are suspected or recognized.
- Intramuscular route for epinephrine therapy remains superior in terms of an initial treatment. Conversely, intravenous route for initial treatment is only recommended in a perioperative setting with IV infusion preferred over bolus doses.
- Optimal dose for IM epinephrine is recommended as 0.5 mg in all adults and 0.01 mg/kg titrated to clinical response in children based on international guidelines.
- In refractory cases, IM epinephrine is recommended to be given every 5 minutes.
- Intravenous epinephrine infusion should be considered when respiratory or cardiovascular symptoms of anaphylaxis persist after 2 appropriate IM doses. In terms of the non-primary management options for anaphylaxis, intravenous fluids are recommended to be infused in the presence of hemodynamic instability.
- Antihistamines and corticosteroids remain two of the most frequently administered drug therapy in anaphylaxis. However, antihistamines are recommended not to be used as part of the initial emergency treatment. Corticosteroids are also recommended not to be routinely used in treating anaphylaxis but only as a third-line intervention for treating underlying asthma or shock.
- Lastly, beta-2 agonists are recommended to be considered adjunct treatment for lower respiratory symptoms after appropriate management with IM epinephrine initially.
References
- Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organization Journal. 2020;13(10):100472. doi:10.1016/j.waojou.2020.100472
- Wood RA, Camargo CA, Lieberman P, et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. Journal of Allergy and Clinical Immunology. 2014;133(2):461-467. doi:10.1016/j.jaci.2013.08.016
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: Guidelines from the European Academy of allergy and Clinical Immunology. Allergy. 2014;69(8):1026-1045. doi:10.1111/all.12437
- Dunn JD, Sclar DA. Anaphylaxis: A PAYOR'S perspective on epinephrine autoinjectors. The American Journal of Medicine. 2014;127(1). doi:10.1016/j.amjmed.2013.09.013
- Campbell RL, Li JTC, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: A practice parameter. Annals of Allergy, Asthma & Immunology. 2014;113(6):599-608. doi:10.1016/j.anai.2014.10.007
- Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ. Evidence update for the treatment of anaphylaxis. Resuscitation. 2021;163:86-96. doi:10.1016/j.resuscitation.2021.04.010
- Australasian Society of Clinical Immunology and Allergy (ASCIA). Guideline for the acute management of anaphylaxis; 2020. https://www.allergy.org.au/hp/papers/acute-management-of-anaphylaxis-guidelines.
- Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 PRACTICE parameter UPDATE, systematic review, and grading of Recommendations, Assessment, development and Evaluation (GRADE) ANALYSIS. Journal of Allergy and Clinical Immunology. 2020;145(4):1082-1123. doi:10.1016/j.jaci.2020.01.017
- Simons FE, Ardusso LRF, Bilò MB, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organization Journal. 2011;4(2):13-37. doi:10.1097/wox.0b013e318211496c
- Soar J, Pumphrey R, Cant A, et al. Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation. 2008;77(2):157-169. doi:10.1016/j.resuscitation.2008.02.001
- de Silva D, Singh C, Muraro A, et al. Diagnosing, managing and preventing anaphylaxis: Systematic review. Allergy. 2020;76(5):1493-1506. doi:10.1111/all.14580
- Patel N, Chong KW, Yip AYG, et al. Use of multiple epinephrine doses in anaphylaxis: A systematic review and meta-analysis. Journal of Allergy and Clinical Immunology. 2021. doi:10.1016/j.jaci.2021.03.042
- Ko BS, Kim JY, Seo D-W, et al. Should adrenaline be used in patients with hemodynamically stable anaphylaxis? Incident case control study nested within a retrospective cohort study. Scientific Reports. 2016;6(1). doi:10.1038/srep20168
- Liu X, Lee S, Lohse CM, Hardy CT, Campbell RL. Biphasic Reactions in Emergency Department Anaphylaxis Patients: A Prospective Cohort Study. J Allergy Clin Immunol Pract. 2020;8(4):1230-1238. doi:10.1016/j.jaip.2019.10.027
- Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: A systematic review and meta-analysis. The Journal of Allergy and Clinical Immunology: In Practice. 2015;3(3):408-416.e2. doi: 10.1016/j.jaip.2014.12.010.
- Kraft M, Hofmeier KS, Ruëff F, et al. Risk factors and characteristics of biphasic anaphylaxis. The Journal of Allergy and Clinical Immunology: In Practice. 2020;8(10):3388-3395.e6. doi: 10.1016/j.jaip.2020.07.036.
- Turner PJ, Allen KJ, Mehr S, Campbell DE. Knowledge, practice, and views ON precautionary allergen labeling for the management of patients with IgE-mediated food ALLERGY—A survey of Australasian and UK health care professionals. The Journal of Allergy and Clinical Immunology: In Practice. 2016;4(1). doi:10.1016/j.jaip.2015.09.003
- Dreborg S, Kim H. The pharmacokinetics of epinephrine/adrenaline autoinjectors. Allergy Asthma Clin Immunol. 2021;17(1):25. doi: 10.1186/s13223-021-00511-y.
- Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: Higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. The Journal of Allergy and Clinical Immunology: In Practice. 2015;3(1):76-80. doi:10.1016/j.jaip.2014.06.007.
- Simons FER, Gu X, Silver NA, Simons KJ. EpiPen Jr versus EpiPen in young children weighing 15 to 30 kg at risk for anaphylaxis. Journal of Allergy and Clinical Immunology. 2002;109(1):171-175. doi:10.1067/mai.2002.120758
- Duvauchelle T, Robert P, Donazzolo Y, et al. Bioavailability and cardiovascular effects of Adrenaline administered by Anapen Autoinjector in healthy volunteers. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6(4):1257-1263. doi:10.1016/j.jaip.2017.09.021
- Suwan P, Praphaiphin P, Chatchatee P. Randomized comparison of caregivers' ability to use epinephrine autoinjectors and prefilled syringes for anaphylaxis. Asian Pac J Allergy Immunol. 2018;36(4):248-256. doi: 10.12932/AP-020318-0275.
- Turner PJ, Ruiz-Garcia M, Durham SR, Boyle RJ. Limited effect of intramuscular epinephrine on cardiovascular parameters during peanut-induced anaphylaxis: An observational cohort study. J Allergy Clin Immunol Pract. 2021;9(1):527-530.e1. doi: 10.1016/j.jaip.2020.08.041.
- Ruiz-Garcia M, Bartra J, Alvarez O, et al. Cardiovascular changes during peanut-induced allergic reactions in human subjects. Journal of allergy and clinical immunology. 2021;147(2):633-642. doi: 10.1016/j.jaci.2020.06.033.
- Smith PL, Kagey-Sobotka A, Bleecker ER, et al. Physiologic manifestations of HUMAN anaphylaxis. Journal of Clinical Investigation. 1980;66(5):1072-1080. doi:10.1172/jci109936
- Brown SG. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emergency Medicine Journal. 2004;21(2):149-154. doi:10.1136/emj.2003.009449
- Cardona V. Cabañes N. Chivato T. et al. Spanish Society of Allergology and Clinical Immunology (SEAIC), Guía de actuación en ANAFILAXIA: GALAXIA. 2016 https://doi.org/10.18176/944681-8-6
- Alviani C, Burrell S, Macleod A, et al. Anaphylaxis Refractory to intramuscular adrenaline during in-hospital food challenges: A case series and proposed management. Clin Exp Allergy. 2020;50(12):1400-1405. doi:10.1111/cea.13749
- Dhami S, Panesar SS, Roberts G, et al. Management of anaphylaxis: A systematic review. Allergy. 2014;69(2):168-175. doi: 10.1111/all.12318.
- Nurmatov UB, Rhatigan E, Simons FE, Sheikh A. H2-antihistamines for the treatment of anaphylaxis with and without shock: a systematic review. Ann Allergy Asthma Immunol. 2014;112(2):126-131. doi:10.1016/j.anai.2013.11.010
- Ellis BC, Brown SGA. Parenteral antihistamines cause hypotension in anaphylaxis. Emerg Med Australas. 2013;25(1):92-93. doi: 10.1111/1742-6723.12028.
- Gabrielli S, Clarke A, Morris J, et al. Evaluation of prehospital management in a canadian emergency department anaphylaxis cohort. The Journal of Allergy and Clinical Immunology: In Practice. 2019;7(7):2232-2238.e3. doi: 10.1016/j.jaip.2019.04.018.
- Wiley E., Romo N. The association of antihistamine administration and delayed presentation for care in pediatric patients admitted with anaphylaxis. Pediatrics. 2020; 146: 236-238. doi: https://doi.org/10.1542/peds.146.1_MeetingAbstract.236
- Alqurashi W, Ellis AK. Do corticosteroids prevent biphasic anaphylaxis? The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(5):1194-1205. doi: 10.1016/j.jaip.2017.05.022.
- Huang F, MD, Chawla K, MD, Järvinen, Kirsi M., MD, PhD, Nowak-Węgrzyn A, MD. Anaphylaxis in a new york city pediatric emergency department: Triggers, treatments, and outcomes. Journal of allergy and clinical immunology. 2011;129(1):162-168.e3. doi: 10.1016/j.jaci.2011.09.018.
- Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M. Anaphylaxis in an emergency setting – elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012;67(11):1451-1456. doi: 10.1111/all.12012.
- Fineman SM. Optimal treatment of anaphylaxis: Antihistamines versus epinephrine. Postgraduate Medicine. 2014;126(4):73-81. doi: 10.3810/pgm.2014.07.2785.
- Ruiz Oropeza A, Lassen A, Halken S, Bindslev-Jensen C, Mortz CG. Anaphylaxis in an emergency care setting: A one year prospective study in children and adults. Scand J Trauma Resusc Emerg Med. 2017;25. doi: 10.1186/s13049-017-0402-0.
- Dubus J-C, Lê M-S, Vitte J, et al. Use of epinephrine in the emergency department depends on anaphylaxis severity in children. European journal of pediatrics. 2019;178(1):69-75. doi:10.1007/s00431-018-3246-3
- Choi YJ, Kim J, Jung JY, Kwon H, Park JW. Underuse of epinephrine for pediatric anaphylaxis victims in the emergency department: A population-based study. Allergy, asthma & immunology research. 2019;11(4):529-537. doi:10.4168/aair.2019.11.4.529.
- Choo KJL, Simons FER, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev. 2012;2012(4). doi:10.1002/14651858.CD007596.pub3.
- Campbell DE. Anaphylaxis management: Time to re-evaluate the role of corticosteroids. The journal of allergy and clinical immunology in practice (Cambridge, MA). 2019;7(7):2239-2240. doi: 10.1016/j.jaip.2019.07.005.
- Fischer D, Vander Leek TK, Ellis AK, Kim H. Anaphylaxis. Allergy, asthma, and clinical immunology. 2018;14(S2):1-8. doi: 10.1186/s13223-018-0283-4.
- Rainbow J, Browne G. Fatal asthma or anaphylaxis? Emerg Med J. 2002;19(5):415-417. doi:10.1136/emj.19.5.415.
- Payus AO, Ibrahim A, Mustafa N. “Two stones on one bird”: A case report on severe biphasic anaphylaxis masquerading as life-threatening acute asthma. Open Access Maced J Med Sci. 2018;6(11):2136-2138. doi:10.3889/oamjms.2018.317.