Article
CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713-1723.
Tranexamic acid (TXA) has received much attention over the past decade secondary to its usefulness in treating hemorrhage. Released in October 2019, CRASH-3 is the latest trial exploring the benefits of TXA, this time in traumatic brain injury (TBI).1 Each year there are nearly 70 million cases of traumatic brain injury worldwide,2 and in the United States more than 2.5 million patients seek care in the ED for TBI every year, accounting for approximately 2% of all ED visits.3 CRASH-3 evaluated the effect of TXA on 28-day head injury-related death in patients with acute TBI.
BACKGROUND
Before diving into the study itself, it may be useful to briefly review why TXA has been so heavily investigated as a pharmacologic tool to help control hemorrhage. Having been accepted for the better part of two decades amongst the surgical community due to its reduction of peri-operative blood transfusion requirements, TXA is an anti-fibrinolytic agent that works by displacing plasminogen from fibrin to stabilize and inhibit clot breakdown. In the case of trauma, hyperfibrinolysis has long been an associated component of the coagulopathy of trauma. When present, mortality has been observed to be in excess of 70%.4,5,6 Particularly in the case of severe, blunt trauma, tissue hypoperfusion stimulates the release of tissue-plasminogen activator (t-PA) from vascular endothelial cells, resulting in fibrinolysis and impeding hemostasis.7 It is the role of TXA to directly inhibit this process. While it may be intuitive then to infer that the benefits of TXA may only be limited to those in shock, it is important to note that hyperfibrinolysis has been an identified component in severe trauma, including isolated head trauma, even when the clinical course was not complicated by hypotension.8 Accordingly, if similar hemostatic benefits seen in surgical patients are extended to trauma patients, TXA may offer a low-cost, low-risk treatment option to impact outcomes in trauma surgery.
Over the past decade, multiple trials have investigated the usefulness of TXA in hemorrhage. Published in 2010, CRASH-2 was a massive RCT with more than 20,000 patients, which showed a 1.5% decrease in mortality when TXA was given to trauma patients with significant bleeding. This effect was time dependent and only seen when given within 3 hours and increasing to 2.1% when given within 1 hour. There was no observed increase in thromboembolic events. However, a subgroup analysis showed there was an increase in death due to bleeding when given >3 hours from injury.9 The Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study published in 2012 included 896 combat injuries treated in Afghanistan in which the TXA group had a 6.5% mortality benefit over placebo despite being a more severely injured group.10 The WOMAN trial published in 2017 studied the effects of TXA in postpartum hemorrhage in over 20,000 patients. This study showed a statistically significant 0.5% reduction in death due to postpartum hemorrhage when given within 3 hours of birth as a secondary outcome with no increase in thromboembolic events, but no significant decrease in the primary outcome of all-cause mortality with its use.11 Finally, The Tranexamic acid for hyperacute primary IntraCerebral Hemorrhage (TICH-2) trial released in 2018 studied the effect of TXA on non-traumatic ICH when given within 8 hours. This study showed no benefit in the primary endpoint of functional status at 90 days or secondary outcomes of hematoma expansion; however, it did show a significant reduction in early deaths at day 7.12
What did they do?
This brings us to the latest evolution of TXA research: CRASH-3. This was a very large, pragmatic, double-blinded RCT conducted at 175 hospitals in 29 countries across a range of geographic and economic settings (although no North American centers participated) from 2012-2019. Included patients were adults with TBI treated within 3 hours of injury who had a GCS ≤ 12 or any intracranial bleeding on CT (inclusion was originally within 8 hours; however, this was changed in 2016 after data showed patients were less likely to benefit from TXA when given beyond that time point). Patients were excluded if they had any obvious major extracranial bleeding. The intervention was 1g TXA given over 10 minutes followed by a 1g infusion over 8 hours versus a matching placebo regimen.
The primary outcome in this study was disease-specific being head injury-related death within 28 days in those treated within 3 hours. Notable secondary outcomes included early head injury-related death (<24 hours), all-cause and cause-specific mortality, disability, vascular occlusive events (MI, CVA, DVT, and PE) and seizure. Of note, the authors had a pre-specified sensitivity analysis that excluded patients with a GCS of 3 or bilateral unreactive pupils as these patients were expected to have a very poor prognosis regardless of treatment and may bias treatment effect towards the null.
RESULTS
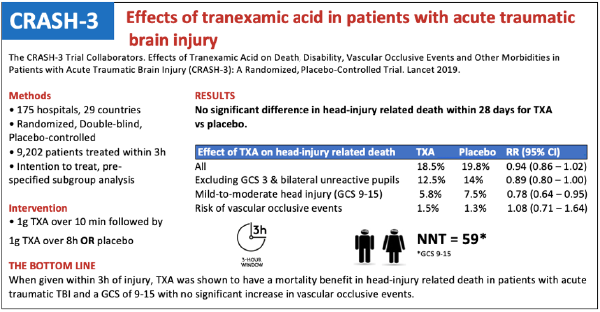
Of the 12,737 patients randomized, 9,202 were treated within 3 hours of injury. Baseline characteristics including sex, age, time since injury, systolic blood pressure, GCS, and pupil reactivity were well-matched at randomization. As for the primary outcome, there was no statistical difference in head injury-related death within 28 days of injury with TXA compared to placebo (TXA 18.5% versus placebo 19.8%; RR .94; 95% CI 0.86 – 1.02). Similarly, there was no statistically significant difference between TXA and placebo in the pre-specified sensitivity analysis which removed patients with GCS of 3 or bilateral unreactive pupils (TXA 12.5% versus placebo 14%; RR 0.89; 95% CI 0.80 – 1.00).
This brings us to the subset of patients receiving the most attention following the publication of the trial. A subgroup analysis for risk of head injury related death in patients with mild-to-moderate head injury (GCS 9-15) was significantly reduced (5.8% vs 7.5%; RR.78; 95% CI 0.64 – 0.95; NNT 59). In this subgroup, early treatment was also found to be more effective than later treatment (p=0.005). The benefit did not carry over to those with severe head injury (GCS 3-8; RR .99; 95% CI 0.91 – 1.07).
As for the secondary outcomes, TXA was found to be generally safe with the risk of vascular occlusive events similar in both groups (RR .98; 95% CI 0.74 – 1.28) and there was no observed increase in the risk of seizures (RR 1.09; 95% CI 0.90 – 1.33). Of note, TXA did increase the relative risk for non-head injury related death, however this too was not shown to be statistically significant (RR 1.31; 95% CI 0.93 – 1.85). Finally, among survivors there was no improvement in patient-centered disability for TXA vs placebo as determined by two separate disability measures.
DISCUSSION
How should CRASH-3 be interpreted and what are the takeaways? The primary outcome of this study may be considered negative, with no significant difference seen between head injury-related death within 28 days when comparing TXA vs placebo given within 3 hours. However, when looking at the data more closely there appears to be a subset of patients for which TXA has a definitive benefit.
When excluding patients with a GCS of 3 or bilaterally unreactive pupils, those who are likely too sick to benefit from any intervention are removed, a dilutional skew towards a negative outcome is taken out. Patients with a GCS of 9-15 are less sick on presentation, but also have more room to decompensate following their TBI. This is the subset of patients where TXA was shown to have the highest mortality benefit. It may be that this benefit was simply not large enough to affect the mortality endpoints for the entire patient population studied, resulting in a negative primary endpoint. One can also conclude that earlier treatment is more effective than later treatment in patients with mild to moderate TBI, a similar finding to previously published literature regarding the need for timely administration of TXA in hemorrhage. Thus, increasing delays in TXA administration would reduce the potential for therapy to prevent expansion of intracranial hemorrhage in TBI.
Several limitations should also be mentioned. These include quite wide confidence intervals, making both a substantial and little to no benefit possible, a disease specific primary outcome which may be affected by misclassification of cause of death (although this was blinded to trial treatment), and possible underestimation of DVT/PE.
The authors summarized their findings by stating “tranexamic acid is safe in patients with TBI and that treatment within 3 h of injury reduces head injury-related deaths.” A caveat could be made this applies most clearly to patients with a GCS of 9-15. With a now demonstrated mortality benefit in TBI following CRASH-3 combined with the low cost and thus far proven safety of TXA, it is certainly reasonable to include timely administration of TXA to more established interventions (elevating the head of the bed to 30°, blood pressure control, avoidance of hypoxia, etc.) as part of the treatment regimen for patients presenting to the emergency department with acute TBI.
REFERENCES
- Dewan Y, Komolafe EO, Mejia-Mantilla JG, Perel P, Roberts I, Shakur H. CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87.
- Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;April1:1-18.
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(No. SS-9):1-16.
- Ives C, Inaba K, Branco BC, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215(4):496-502.
- Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365-370; discussion 370.
- Schöchl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125-131.
- Nishida T, Kinoshita T, Yamakawa K. Tranexamic acid and trauma-induced coagulopathy. J Intensive Care. 2017 Jan 20;5:5.
- Hayakawa M. Pathophysiology of trauma-induced coagulopathy: disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017;5:14.
- Perel P, Al-Shahi SR, Kawahara T, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) Intracranial Bleeding Study: The effect of tranexamic acid in traumatic brain injury - a nested, randomised, placebo-controlled trial. Health Technol Assess. 2012;16(13):iii-xii,1-54.
- Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147(2):113-119.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084), 2105–2116.
- Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107–2115.




