Article
Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;Sept. 2;online-first.
Objective
To identify the effect of dexamethasone on ventilator-free days in patients with COVID-19 associated ARDS.
Background
With the publication of the RECOVERY Trial in June, the steroid dexamethasone was the first drug identified to reduce mortality in COVID-19 patients. Dexamethasone had the largest mortality benefit in mechanically ventilated patients and those on ECMO, followed by those with an oxygen requirement. This suggests that it provides a greater benefit in sicker patients.
Acute Respiratory Distress Syndrome (ARDS) is a disease entity seen in critical patients that stems from inflammatory dysregulation in the lungs after a physiologic insult that leads to hypoxic respiratory failure. The DEXA-ARDS Trial, published earlier this year, demonstrated that dexamethasone can reduce mortality and ventilator free days in patients with moderate to severe ARDS.
Severe COVID-19 infection is often associated with ARDS. As steroids have been shown to reduce mortality in both COVID-19 patients and in ARDS patients, it is likely that they will be beneficial for patients with COVID-19 associated ARDS. The CoDEX Trial attempts to determine the effect of steroids in these patients.
Design
Multicenter, open-label, randomized control trial at 41 ICUs in Brazil
Population
Inclusion Criteria:
- 18 years of age or older
- Suspected or confirmed infection
- Receiving mechanical ventilation
- Had moderate to severe ARDS, defined according to the Berlin Criteria as a PaO2:FiO2 ratio of 200 or less
Exclusion Criteria:
- Pregnancy or active lactation
- Corticosteroid use in the past 15 days or during the hospital stay for more than 1 day
- Other medical condition necessitating steroids
- Use of immunosuppressants or cytotoxic chemotherapy for malignancy
- Expected death within 24 hours
Intervention
Dexamethasone 20 mg IV once daily for 5 days, then 10 mg IV for 5 days or until ICU discharge, whichever came first
Control
Standard care. Non-study corticosteroids were permitted for other ICU indications, such as bronchospasm or septic shock
Primary Outcome
- Ventilator-free days in the first 28 days, defined as days alive and free of mechanical ventilation for at least 48 hrs
Secondary Outcomes
- 28-day all-cause mortality
- Clinical status of patients at day 15 using a WHO 6-point ordinal scale
- ICU-free days in the first 28 days
- Mechanical ventilation duration at 28 days
- SOFA scores at 48 hrs, 72 hrs, and 7 days
Key Results
The CoDEX trial was terminated early due to ethical concerns after the publication of the successful RECOVERY Trial on June 25, 2020 after randomizing 299 patients of a planned 350. Due to the open-label nature of the trial, compliance was tracked closely. Only one patient in the intervention group did not receive dexamethasone, but 35% of control group patients received a steroid. About 10% of control group patients received steroids without another clinical indication, which was considered a protocol violation. Results were adjusted for age and PaO2:FiO2 ratio at randomization.
Days alive and free of a ventilator in 28 days was 6.6 days in the dexamethasone group and 4.0 days in the control group, for an adjusted difference of 2.26 days (95% CI: 0.2-4.38, P= 0.04).
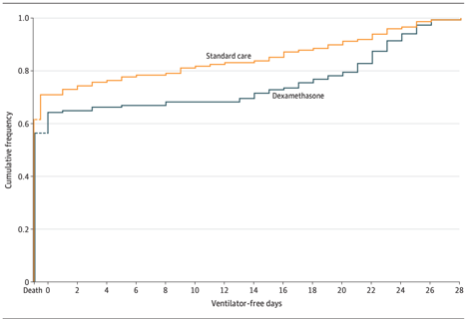
There was no difference in secondary outcomes or adverse effects between the groups.
Strengths
This is a well-run, randomized controlled trial that focused on very sick COVID-19 patients. The researchers followed the patients closely and conducted multiple sensitivity analyses to ensure validity despite the open-label nature of the trial.
Limitations
The major limitation of this trial is that it was not blinded. The authors state that this was because they could not have a placebo produced in time during the pandemic. In the end, 10% of the control group received at least some part of the intervention, presumably because treating physicians knew their allocation and wanted to provide the intervention to the control group patients. These violations bias the study to underestimate the effect size, and thus should not negate the final positive outcome. The unblinded nature, however, may have biased the reporting of adverse events.
This study was also stopped early after the publication of the RECOVERY Trial. This also biases towards the null and should not cast doubt on the ultimate positive outcome, but it means that the secondary outcomes were underpowered to find an effect.
In addition, this trial was conducted relatively early in the outbreak in Brazil, so Remdesivir and other COVID-19 treatments were not available to patients in the study. One should be careful extrapolating these results to the treatment of patients receiving other COVID therapies.
EM Take-Aways
This paper, along with those released with it, provide strong evidence that steroids are beneficial for COVID-19 patients. The CoDEX Trail, in particular, expands on the RECOVERY Trial to demonstrate that steroids are beneficial in particularly sick COVID-19 patients with high expected mortality. The patients in this trial had approximately a 60% mortality rate. This rate is higher than seen in non-COVID-19 ARDS patients, but seems to approximate the mortality rate seen in COVID-19 related ARDS in South America. The authors quote Brazilian mechanically ventilated COVID-19 ICU mortality rates between 66-70%. The authors also point out that in addition to direct benefits to patients, small reductions in days on a ventilator can provide large benefits to overburdened healthcare systems during the global pandemic, freeing up ventilators for other patients sooner.




