Article
Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. Online ahead of print. Nov. 13, 2020.
Objective
To compare survival to hospital discharge between standard ACLS resuscitation and early ECMO facilitated resuscitation for cardiac arrest with refractory ventricular fibrillation.
BACKGROUND
Out-of-hospital cardiac arrest accounts for 350,000 deaths yearly and the presenting rhythm for any given patient provides insight into their possible survivability to hospital discharge.1 Between 60-80% of patients who do survive initially present in a shockable rhythm such as ventricular fibrillation (VFib) or pulseless ventricular tachycardia. Patients who present in pulseless electrical activity (PEA) or asystole are almost all likely to die. Shockable rhythms are believed to be an indicator of ischemic pathology that may be reversible, i.e. coronary artery disease. Different modalities continue to be investigated for refractory shockable rhythms, including double sequential defibrillation and ECMO facilitated resuscitation. In the ARREST trial, the investigators present evidence of what is possible with ECMO facilitated cardiopulmonary resuscitation for patients who present from out of hospital cardiac arrest with a refractory shockable rhythm.
DESIGN
Phase 2, single centre, open-label, adaptive, safety and efficacy randomized clinical trial
POPULATION
Inclusion Criteria:
- 18-75 years old
- Initial OHCA rhythm of ventricular fibrillation or pulseless ventricular tachycardia
- No ROSC after three defibrillation shocks
- Body morphology able to accommodate Lund University Cardiopulmonary Assist System (LUCAS)
- Estimated transfer time < 30 mins
Exclusion Criteria:
- Valid DNR order
- Blunt, penetrating, or burn related injury
- Drowning
- Known overdose
- Pregnancy
- Prisoner status
- Nursing home resident
- Presence of opt-out bracelet
- Unavailability of catheterisation laboratory
- Terminal cancer
- Absolute contraindications to emergent angiography
- Contrast allergy
- Active gastrointestinal or internal bleeding
- Sustainable ROSC within first 3 shocks
INTERVENTION
Patients in the intervention group were randomized to receive early veno-arterial ECMO support with immediate access to cardiac catheterisation lab regardless of presence or absence of pulses on hospital arrival. Patients then had an arterial blood gas obtained and resuscitation was terminated if two or more of the following were met: end tidal CO2 <10 mm Hg, PaO2 < 50 mm Hg or oxygen saturation <85%, and lactic acid >18 mmol/L). If not, the patient underwent angiography and revascularization as clinically indicated.
Full intervention details and reasoning can be found here: Rationale and methods of the Advanced R2Eperfusion STrategies for Refractory Cardiac Arrest (ARREST) trial
CONTROL
Patients in the control group were randomized to receive standard ACLS resuscitation.
OUTCOMES
Primary Outcome:
- Survival to hospital discharge
Secondary Outcomes:
- Safety, survival, and functional assessment at hospital discharge and at 3 months and 6 months after discharge
KEY RESULTS
Primary Outcome
- Survival to hospital discharge
- 6 of 14 patients in the ECMO group survived (43%, 21.3–67.7) vs 1 of 15 patients in the standard ACLS group (7%,1.6–30.2)
- Risk difference: 36% (3.7–59.2; posterior probability=0.9861)
- 1 survivor in ACLS group who did not survive to 3 month follow-up
Secondary Outcomes
- Survival at 3 months
- 6 of 14 patients in the ECMO group (43%, 21·3–67·7) vs 0 of 15 patients in the standard ACLS group (0·0–20·4)
- Survival at 6 months
- 6 of 14 patients in the ECMO group (43%, 21·3–67·7) vs 0 of 15 patients in the standard ACLS group (0·0–20·4)
- Cerebral Performance Category (CPC)
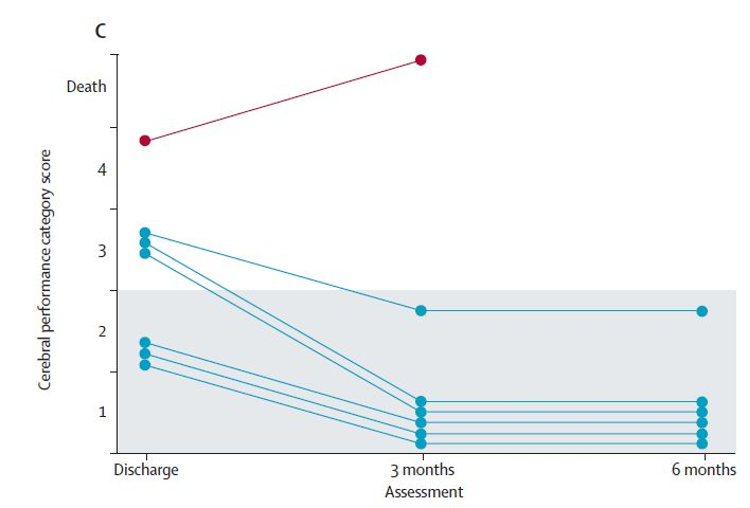
- A lower score on this scale represents better neurological and functional status
- Overall, patient function improved from hospital discharge to the 6 month follow up in the ECMO group
- Hospital Discharge
- 5 (0.5) in the ECMO group vs 4 in the isolated survivor in the standard ACLS group
- 3 Months
- 16 (0·4) in the ECMO group, no survivors in ACLS group
- 6 Months
- 16 (0.4) in the ECMO group, no survivors in ACLS group
- Modified Rankin Scale (mRS)
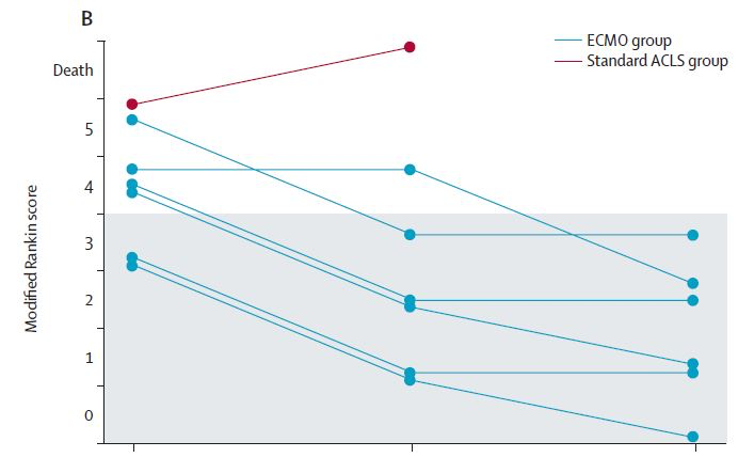
- A lower score on this scale represents better functional status neurologically and physically
- Overall, patient function improved from hospital discharge to the 6 month follow up in the ECMO group
- Hospital Discharge
- Average 3.8 (0.7) in the ECMO group vs 5 in the isolated survivor in the standard ACLS group
- 3 Months
- Average 2 (1.2) in the ECMO group, no survivors in ACLS group
- 6 Months
- 3 (0.8) in the ECMO group, no survivors in ACLS group
STRENGTHS
- Demonstrates the impact of a highly organized and sophisticated pre-hospital system coordinated with critical care interventional cardiology team
- Specific inclusion criteria captured the ideal patient population that would benefit from ECMO facilitated resuscitation
- Prespecified analysis at set points in data collection and participant recruitment
- Survivors had improving mRS and CPC scores associated with minimal symptoms or deficits and good cerebral function at 6 months
- Completeness of data and follow up, no loss of participants in ECMO group
LIMITATIONS
- Single center, prevents external validity
- Quality of pre-hospital system prevents broader application
- Presence of a highly specialized interventional critical care cardiology team is not necessarily feasible or practical in all areas
- Small study numbers
- Coordination of ECMO to be done in cardiac cath lab is ideal, however may not be applicable broadly
EM TAKE-AWAYS
This study presents what is possible with highly coordinated ECMO facilitated cardiopulmonary resuscitation in the ideal patient population presenting with out of hospital cardiac arrest and refractory shockable rhythm. With access to ECMO facilitated resuscitation, the next consideration in developing any protocol is the sophistication of the pre-hospital system and associated transport times. For urban centers with a large catchment area and shorter transport times, it is worth considering development of an ECMO facilitated resuscitation protocol for patients presenting in refractory shockable rhythms. This study also provides support for exploring interesting alternatives such as ECMO cannulation in the emergency department to facilitate earlier access.
Further Reading Recommendations
The Bottom Line (UK) Summary
REFERENCES
- Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(suppl 2):S444–64.
- Yannopoulos D, Bartos JA, Aufderheide TP, et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139:e530–52.