In the United States, more than 2.5 million patients seek care in the ED for traumatic brain injury (TBI) every year, accounting for approximately 2% of all ED visits.1 Caring for TBI in the ED can be a daunting task, as there are numerous variables to consider, and care recommendations are constantly evolving.
In this article, we revisit the equation for cerebral perfusion pressure (CPP) as a simplified framework to approach TBI care in the ED. Optimization of CPP is necessary to meet the metabolic needs of the injured brain, prevent secondary injury, and minimize future disability.2 The equation is as follows:
Cerebral perfusion pressure = Mean arterial pressure — Intra-cranial pressure (CPP = MAP -ICP)3
The current goal CPP is thought to be between 60 mmHg and 70 mmHg.2,4,5 In this equation, we can see that CPP is contingent on the pressure gradient between MAP and ICP. By evaluating our strategies for controlling MAP and ICP, we can form a conceptual framework for management of TBIs in the ED.
Mean Arterial Pressure
In TBI, it is important to avoid both hypotension and hypertension, which result in cerebral ischemia and vasogenic edema, respectively.4 For patients with severe TBI, early ICP monitoring should be obtained in consultation with a neurosurgeon to allow for titration of the patient’s blood pressure (BP) to target CPP goals. Before ICP monitoring is in place, it is recommended to maintain MAP between 80 mmHg and 110 mmHg.4
Manage hypertension with titratable, short-acting beta-blockers and calcium channel blockers such as nicardipine, clevidipine, and esmolol.6
Treat hypotension with fluid boluses or vasopressors such as norepinephrine, epinephrine, and phenylephrine. Identify and address underlying causes of hypotension, such as hypovolemic or neurogenic shock.
Although airway management may be necessary for severe TBI, intubation can be accompanied by undesirable hemodynamic changes. Laryngoscopy and tracheal manipulation lead to sympathetic stimulation, which provokes elevation in heartrate and BP. Consider pretreatment with fentanyl to blunt the catecholamine surge resulting from direct laryngoscopy. Lidocaine for pre-treatment has not been shown to have significant effect on ICP, CPP, or MAP in response to laryngoscopy.6,7,8,9
Conversely, airway management can also lead to hypotension due to hemodynamic effects of induction agents, sedation agents, and positive pressure ventilation. Close monitoring, judicious medication selection, and optimization of MAP prior to intubation are necessary to avoid negative outcomes.
Induction medications to use in TBI include ketamine, propofol, or etomidate. Ketamine, ostracized in the past due to concern of increased ICP, has re-emerged as a reasonable choice of induction agent. In addition to its favorable hemodynamic profile, a growing body of evidence suggests that ketamine does not adversely affect ICP, CPP, neurologic outcomes, or mortality in TBI patients.6,9,10
Paralysis can be employed with a variety of neuromuscular blocking agents, such as succinylcholine or rocuronium. Succinylcholine has a short duration of onset and can be beneficial for patients requiring frequent neurologic reassessments. However, providers should recall standard contraindications to succinylcholine that may present risk to patients suffering TBI, such as crush injury and prolonged immobilization.6,9
Post-intubation sedation and analgesia is important for a variety of reasons: it minimizes patient anxiety and agitation, reduces the cerebral metabolic rate of oxygen consumption, and facilitates mechanical ventilation.4 Propofol has come into favor as a sedation agent in TBI as it decreases the cerebral metabolic rate and lowers the seizure threshold, as well as being rapidly metabolized to facilitate frequent neurological checks.4,9 Analgesics such as fentanyl, morphine, and hydromorphone may also be used in conjunction with sedative agents.4,9
INTRACRANIAL PRESSURE
The adult skull is an enclosed space and can hold a fixed volume. Therefore, a change in the volume of one of the contents must result in a change in volume of one of the other contents. The normal brain can autoregulate cerebral blood flow within a certain range of MAP and CPP, but in the setting of TBI, autoregulation can become compromised and require intervention.3,6 The goal ICP is <22 mmHg. We can look at the interventions available to providers by going through each of the contents of the skull.
Vessels/Blood
Carbon dioxide causes vasodilation (via H+) of cerebral blood vessels such that PaCO2 has a linear correlation with cerebral blood flow between 20-80 mmmHg.3,10 In intubated patients, it is important to avoid both hyperventilation, which results in ischemia, and hypoventilation, which results in hyperemia.3 Goal PaCO2 is between 35-38 mmHg.9 Hyperventilation to PaCO2<30 mmHg is no longer globally recommended but can be considered in cases where herniation appears imminent.11
Oxygen is necessary for the high metabolic demands of the brain. Maintain SpO2 >95%, as hypoxia has been linked to poor clinical outcomes in TBI patients, particularly in conjunction with hypotension.3 In patients requiring airway management, adequate preoxygenation, apneic oxygenation, and avoidance of prolonged hypoventilation can prevent desaturation during intubation.
Cervical collars inhibit cerebral venous drainage and thereby increase ICP. Aim to clear the C-collar as soon as possible.
Raise the head of the bed to facilitate venous drainage.
Cerebrospinal Fluid
External ventricular drains (EVDs) are inserted to provide continuous or intermittent drainage of CSF, and have shown benefit in TBI patients with a GCS <6.10 These can be done by a neurosurgeon at the bedside in the ED.
Parenchyma
Mannitol or hypertonic saline can be used to decrease cerebral edema. The debate continues as to which is more effective, as the current evidence does not support the use of one over the other in terms of morbidity and mortality outcomes. Arguments toward the use of HTS include greater decreases in ICP, longer duration of action, and lower incidence of acute renal failure and dehydration when compared to mannitol.12,13
Fluid resuscitation should be employed with iso-osmolar fluids such as 0.9% NaCl and PlasmaLyte to avoid cerebral edema.14 Avoid lactated Ringer’s and other hypo-osmolar fluids, such as D5 0.45%NaCl.14
Skull
A decompressive craniectomy may be a reasonable surgical option for the management of severe TBI. To date, there have been two multi-center randomized controlled trials examining morbidity and mortality outcomes in TBI patients with elevated ICP refractory to medical management: DECRA15 and RESCUEicp.16 These trials have demonstrated some conflicting findings, and the effect of decompressive craniectomy in TBI remains unclear. Craniectomies have likely been linked to decreased ICP and reduced mortality in patients with TBI, but they have also been linked to increased survival with vegetative state and severe disability, theorized to be due to axonal stretch as the brain is allowed to stretch outside the bounds of the skull.11,15,16 The decision to perform a craniectomy should involve family members to determine individual values and goals of care.
Burr holes may be employed emergently for suspected or known epidural hematoma with signs of herniation, especially in hospitals without ready access to neurosurgical care.17,18
Conclusion
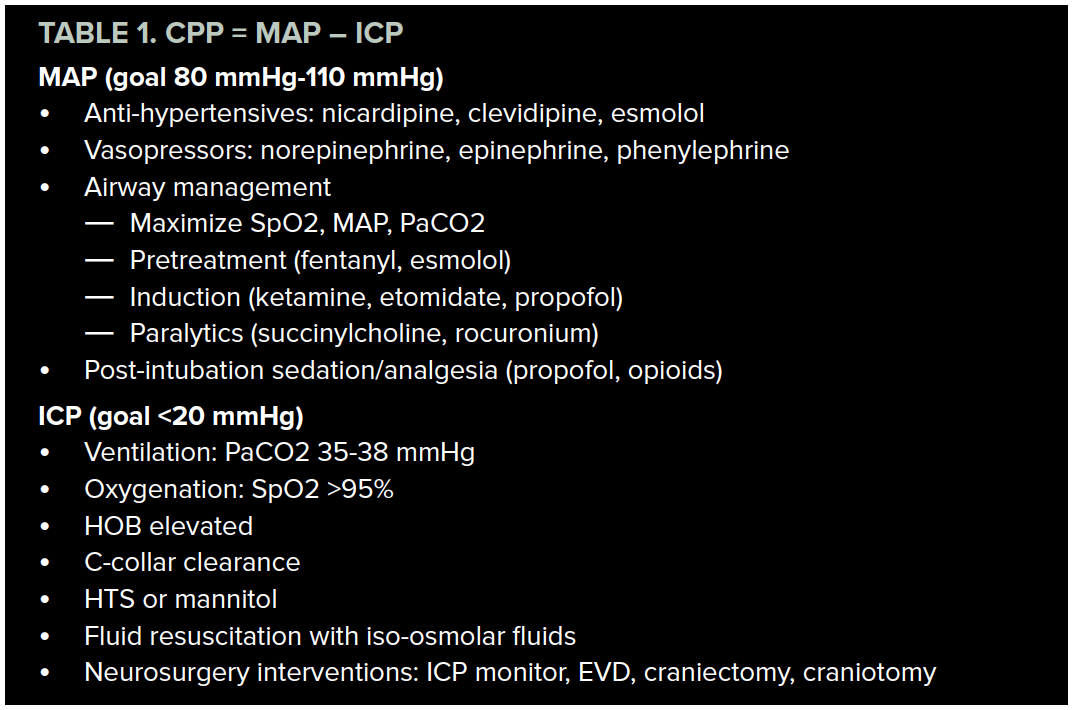
In this article, we have suggested a framework for conceptualizing TBI treatment based on the equation for calculating CPP: CPP = MAP – ICP. We have reviewed management options and treatment goals for MAP and ICP, which are summarized in the table below.
Patients requiring airway management are particularly vulnerable to hemodynamic disturbances and hypoxia, and these potential sources of secondary injury should be anticipated and mitigated.
Early neurosurgery consultation for patients with severe TBI is essential, as these patients may benefit from invasive procedures, such as ICP monitoring, external ventricular drains, or decompressive craniectomy.
References
1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. MMWR Surveill Summ 2017;66(No. SS-9):1–16.
2. Prabhakar H, Sandhu K, Bhagat H, Durga P, Chawla R. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. Journal of Anaesthesiology, Clinical Pharmacology. 2014;30(3):318-327
3. Cipolla MJ. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. Chapter 5, Control of Cerebral Blood Flow.
4. Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. British Journal of Anaesthesia. 2007;99(1):32-42.
5. Carney N, Totten AM, O'Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1;80(1):6-15.
6. Bucher J, Koyfman A. Intubation of the neurologically injured patient. J Emerg Med. 2015;49(6): 920-927.
7. Robinson N, Clancy M. In patients with head injury undergoing rapid sequence intubation, does pretreatment with intravenous lignocaine/lidocaine lead to an improved neurological outcome? A review of the literature. J Emerg Med. 2001;18(6):453-457.
8. Lin CC, Yu JH, Lin CC, Li WC, Weng YM, Chen SY. Postintubation hemodynamic effects of intravenous lidocaine in severe traumatic brain injury. Am J Emerg Med. 2012; 30(9):1782-1787.
9. Rajajee V, Riggs B, Seder DB. Emergency Neurological Life Support: Airway, Ventilation, and Sedation. Neurocrit Care. 2017;27(Suppl 1):4-28.
10. Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM. The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review. Ann Emerg Med. 2015;65:43-51.
11. Griesdale DE, McEwen J, Kurth T, Chittock DR. External ventricular drains and mortality in patients with severe traumatic brain injury. Can J Neurol Sci.2010;37(1):43-48.
12. Kamel H, Navi BB, Nakagawa K, Hemphill JC, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;39(3):554-559.
13. Li M, Chen T, Chen SD, Cai J, Hu YH. Comparison of equimolar doses of mannitol and hypertonic saline for the treatment of elevated intracranial pressure after traumatic brain injury: a systematic review and meta-analysis. Medicine. 2015; 94(17):e736.
14. Alvis-Miranda HR, Castellar-Leones SM, Moscote-Salazar LR. Intravenous Fluid Therapy in Traumatic Brain Injury and Decompressive Craniectomy. Bull Emerg Trauma. 2014;2(1):3-14.
15. Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493-1502.
16. Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119-1130.
17. Nelson J. Local Skull Trephination Before Transfer Is Associated With Favorable Outcomes in Cerebral Herniation from Epidural Hematoma. Acad Emerg Med. 2011;18:78–85
18. Eaton J, Hanif AB, Mulima G, Kajombo C, Charles A. Outcomes Following Exploratory Burr Holes for Traumatic Brain Injury in a Resource Poor Setting. World Neurosurg. 2017;105: 257-264.