ARTICLE:Cheskes S, Verbeek PR, Drennan IR, et al. Defibrillation Strategies for Refractory Ventricular Fibrillation. N Engl J Med. 2022;387:1947-1956.
OBJECTIVE: To evaluate double sequential external defibrillation (DSED) and vector-change defibrillation (VC) as compared with standard defibrillation in patients who remain in refractory ventricular fibrillation during out-of-hospital cardiac arrest.
BACKGROUND
Ventricular fibrillation contributes to a significant portion of out-of-hospital cardiac arrest. Standard guidelines promote early defibrillation for cessation of the arrhythmia. Ventricular fibrillation and pulseless ventricular tachycardia have a higher chance of survival than other cardiac rhythms during cardiac arrest. Despite this, some patients remain in refractory ventricular fibrillation, which is usually defined as persistent ventricular fibrillation after three defibrillation attempts. Previous literature has found that 30-day survival is 28.7% in patients who can be cardioverted in the first three shocks; survival drops to 4.9% among those needing >10 shocks for successful cardioversion.1 Following defibrillation, advanced cardiac life support (ACLS) promotes the use of antiarrhythmics, but no antiarrhythmic has been shown to improve neurologically intact survival.2
Several alternative defibrillator techniques have been proposed. Two of these are DSED and VC defibrillation. In VC defibrillation, the pads are switched from the standard anterior-lateral (AL) to an anterior-posterior (AP) position to change the vector of the electrical shock. This may allow for a higher current to pass through portions of the ventricle not reached by the standard AL position. In DSED, two defibrillators are used. One set is placed in the AL position and the other in the AP configurations. Both shocks are immediately given sequentially. Another mode exists, double simultaneous defibrillation (DSD), in which the pads are in AL and AP configuration but the shocks are delivered simultaneously. These defibrillation techniques have been reported in previous observational studies with varying degrees of success.3 Due to this uncertainty, practice both in and out of hospital have not incorporated these alternative techniques for defibrillation into the current cardiac arrest guidelines.4 In addition, there is a theoretical risk of defibrillator damage with multiple shocks. A further confounder in previous studies is a lack of timing considerations of DSED/DSD/VC as they may have been used as a last resort vs. an early defibrillation technique. This study is a randomized control trial designed to evaluate the early use of DSED and VC techniques in refractory ventricular fibrillation.
DESIGN
This study is a three-group, cluster-randomized controlled trial with cross-over conducted with 6 paramedic services in urban and rural communities with a population of 6.6 million. The trial ran from September 2019 to May 2022 and was interrupted from April 2020 to September 2020 due to the COVID-19 pandemic. It was later terminated in May 2022 from concerns that longer response times due to paramedic staffing shortages were interfering with the timely application of the assigned type of defibrillation.
Randomization of treatment sequences was computer-generated by the coordinating center at the level of paramedic service prior to the start of the trial. Each service had to cross over to each of the treatment groups at least once. The three experimental groups include a control group, a VC group, and a DSED group. Groups differed by type of shock received after patient being in refractory ventricular fibrillation.
Refractory ventricular fibrillation was defined as an initial presenting rhythm of ventricular fibrillation or pulseless ventricular tachycardia that was still present after three consecutive rhythm analyses and standard defibrillators separated by 2-minute intervals of CPR. Of note, shocks by fire agencies were included in the 3-shock limit for the main trial. However, they were not counted in a smaller pilot trial which was also included in the overall analysis.
Paramedics followed AHA guidelines for the treatment of patients in ventricular fibrillation. Chest compressions were performed, rhythm analysis occurred at standard 2-minute intervals, and ventricular fibrillation was determined by manual rhythm analysis. All patients received three defibrillation attempts in the AL position prior to deviation to the trial groups. The patients then received the defibrillation type according to the randomly assigned experimental and control groups. All subsequent defibrillation attempts were delivered according to the assigned groups. For the DSED group, shocks were applied with a short (<1 second) delay to avoid possible defibrillator damage. A single paramedic hit the “shock” button on each defibrillator.
INCLUSION CRITERIA
- Patients ≥ 18 years who had an out-of-hospital cardiac arrest due to presumed cardiac cause with refractory ventricular fibrillation in the study period
EXCLUSION CRITERIA
- Patients with a traumatic cardiac arrest
- Patients with do-not-resuscitate orders
- Patients with cardiac arrest due to drowning, hypothermia, hanging, or suspected drug overdose
PRIMARY OUTCOME
- Survival to hospital discharge in patients presenting with out-of-hospital refractory ventricular fibrillation
SECONDARY OUTCOME
- Termination of ventricular fibrillation is defined as the absence of ventricular fibrillation on further rhythm analysis after defibrillation and a 2-minute interval of CPR
- Return of spontaneous circulation (ROSC), defined as any change in rhythm to an organized rhythm with palpable pulse or documented blood pressure
- Good neurologic outcome at hospital discharge is defined as a modified Rankin scale score of 2 or lower
KEY RESULTS
The final cohort consisted of 405 patients. 136 (33.6%) were assigned to the standard group, 144 (35.6%) to the VC group, and 125 (30.9%) to the DSED group. Of these, 87.7% received the type of defibrillation that had been randomly assigned.
Primary outcome
The primary outcome of survival to hospital discharge occurred in 38 (30.4%) patients in the DSED group, 31 patients (21.7%) in the VC group, and 18 (13.3%) in the standard group. Relative risk of survival was 2.21 (95% CI 1.33-3.67) in the DSED group and 1.71 (95% CI 1.01-2.88) in the VC group compared to the standard group. The overall test for the difference of the primary outcome was significant (P=0.009) for the comparisons among the three groups using the generalized linear model.
The fragility index showed if 9 patients in the DSED or 1 patient in the VC group had not survived to discharge, results would have been rendered nonsignificant.
Secondary outcomes
Termination of ventricular fibrillation occurred in 105 (84%) of patients in the DSED group and 92 patients (67.6%) in the standard group (Relative risk 1.25; 95% CI, 1.09-1.44). ROSC was achieved in 58 (46.4%) of patients in the DSED group and 36 (26.5%) in the standard group (Relative risk 1.72; 95% CI, 1.22 to 2.42). Survival with a good neurologic outcome occurred in 34 (27.4%) patients in the DSED group and 15 (11.2%) patients in the standard group (Relative risk 2.21, 95% CI, 1.26-3.88).
Termination of ventricular fibrillation occurred in 115 (79.9%) of patients in the VC group (Relative risk of 1.18; 95% CI, 1.03-1.36 [vs. standard group]). ROSC was achieved in 51 (35.4%) of patients in the VC group (Relative risk 1.39; 95% CI, 0.97 to 1.99 [vs. standard group]). Survival with a good neurologic outcome occurred in 23 (16.2%) of patients in the VC group (Relative risk 1.48, 95% CI, 0.81-2.71 [vs. standard group]).
LIMITATIONS
- The trial did not achieve the planned sample size. This smaller sample size might overestimate the treatment effect. The study was also briefly interrupted due to the COVID pandemic.
- The trial did not specify a further follow-up time outside of hospital discharge.
- The length of stay distribution across the trial centers is not known.
- Small fragility index of 1 for the VC primary outcome.
- The trial was not completely blinded as paramedics were aware of the assigned defibrillation strategy.
- Majority of patients were enrolled in urban settings which may not be generalized for all settings.
- May have limited external validity and generalizability. This study occurred in Canada with a different healthcare structure than many countries. EMT services have different training and roles.
- Trial was conducted with a high degree of medical oversight and paramedic feedback that may not be seen outside of the study or in other countries.
- Paramedics were also specifically trained not just in the study protocol, but retrained on performing high-quality, metric driven CPR. This level of training may not be generalizeable.
- The trial did not include or evaluate double simultaneous defibrillation.
- Although not discussed in the paper, there are safety concerns for equipment and operational challenges that may appear with alternative defibrillation techniques.5
EM TAKE-AWAYS
DSED and VC defibrillation were found to lead to better survival to hospital discharge over standard defibrillation in out-of-hospital cardiac in refractory ventricular fibrillation. This study is the largest and most rigorous to show benefits of these alternative defibrillation techniques. Further studies are needed, but it would be reasonable to employ DSED and VC defibrillation techniques in the setting of refractory ventricular fibrillation in the prehospital or emergency department setting.
Editor's Note: Thanks to former EMRA Critical Care Committee chair Mark Ramzy, DO, for sharing the following infographic.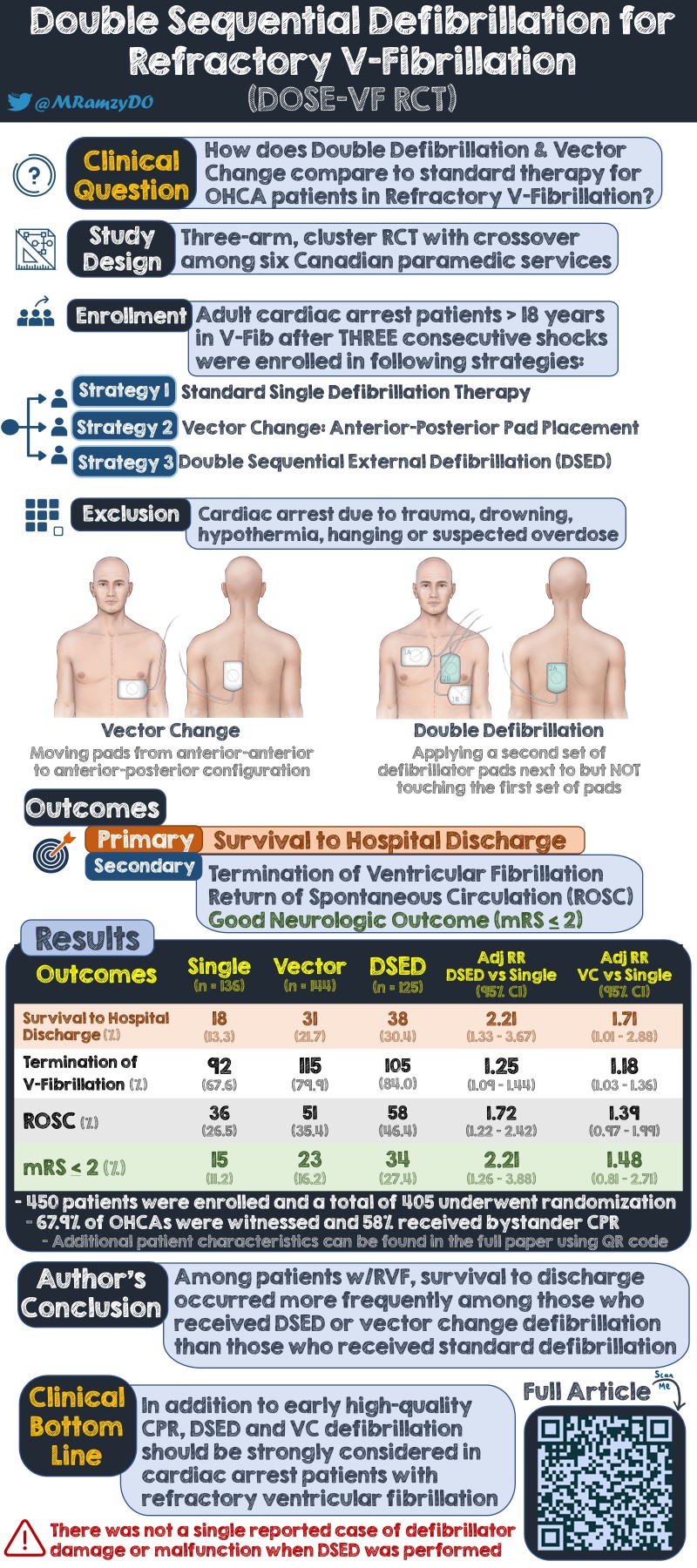
REFERENCES
- Holmén J, Hollenberg J, Claesson A, et al. Survival in ventricular fibrillation with emphasis on the number of defibrillations in relation to other factors at resuscitation. Resuscitation. 2017;113:33-38.
- Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2016;374(18):1711-1722.
- Deakin CD, Morley P, Soar J, Drennan IR. Double (dual) sequential defibrillation for refractory ventricular fibrillation cardiac arrest: A systematic review. Resuscitation. 2020;155:24-31.
- Pourmand A, Galvis J, Yamane D. The controversial role of dual sequential defibrillation in shockable cardiac arrest. Am J Emerg Med. 2018;36(9):1674-1679.
- Gerstein NS, McLean AR, Stecker EC, Schulman PM. External defibrillator damage associated with attempted synchronized dual-dose cardioversion. Ann Emerg Med. 2018;71:109-12.