Case
A 60-year-old male rolls in by EMS with a complaint of shortness of breath. He was discharged from prison two months ago, at which time he ceased all his medications. He believes he was on a “water pill” for his “kidney problem.” He smokes a pack a day. Additionally, he complains of mild chest pain and lower-extremity edema, worse on the left side. He is in moderate distress, tachypneic, tachycardic, and hypoxic with three-word dyspnea. You do not appreciate any JVD, but notice decreased lung sounds at the bases with a diffuse, mild expiratory wheeze. His heart is tachycardic but regular, without murmurs, rubs, or gallops. His abdomen is slightly distended, but soft and non-tender. His lower extremities have 3+ edema on the left and 2+ on the right. His initial EKG is nondiagnostic.
Ultrasound outperforms chest radiograph in nearly every situation when used by trained physicians.
Discussion
Shortness of breath is a common chief complaint. The differential diagnosis is broad and the possibilities range from immediate life threats to those managed as an outpatient. As clinicians, we need a diagnostic tool that enables us to rapidly narrow the differential diagnosis and direct early management. Ideally, this tool will be readily available and have high sensitivities and specificities for the diseases we are most concerned about. For the patient with undifferentiated shortness of breath, ultrasound is that tool. So, while the X-ray tech is in another part of the emergency department and the nurse is attempting intravenous access for the third time, grab the ultrasound machine.
There is useful information to be gained from the chest radiograph, and it has the convenience of not requiring additional acquisition effort on the part of the physician. However, ultrasound outperforms chest radiograph in nearly every situation when used by trained physicians. Ultrasound is the ideal modality for evaluating for pleural effusions, pericardial effusions with tamponade, and deep vein thromboses.1 Echocardiogram can screen for heart failure, wall motion abnormalities, and right heart strain. For pneumothorax, chest X-ray has a sensitivity of 40% and specificity of 99%, compared to bedside ultrasound, which has a sensitivity of 79% and a specificity of 98%.2,3 For pneumonia, chest X-ray has a sensitivity of 67% and specificity of 85% whereas ultrasound has a sensitivity of 98% and a specificity of 95%.4 Additionally, ultrasound can evaluate for response to therapy in real time. Ultrasound can demonstrate improvement of pulmonary edema, resolution of a pneumothorax, as well as minute-to-minute changes in cardiac function.5
There are a number of manuscripts in the literature describing how to use point-of-care ultrasound (POCUS) for the patient with undifferentiated shortness of breath. Lichtenstein, et al, created an algorithm titled “The Blue Protocol” that can be used with lung ultrasound to differentiate between etiologies.6 However, this protocol did not include echocardiogram as part of the evaluation, and for the novice sonographer the algorithm can be cumbersome. In another publication, Drs. Nagdev and Mantuani demonstrated how analyzing the heart, lungs, and IVC with ultrasound can differentiate heart failure from chronic obstructive pulmonary disease (COPD).7 Combining and simplifying these strategies creates a logical and high-yield approach.
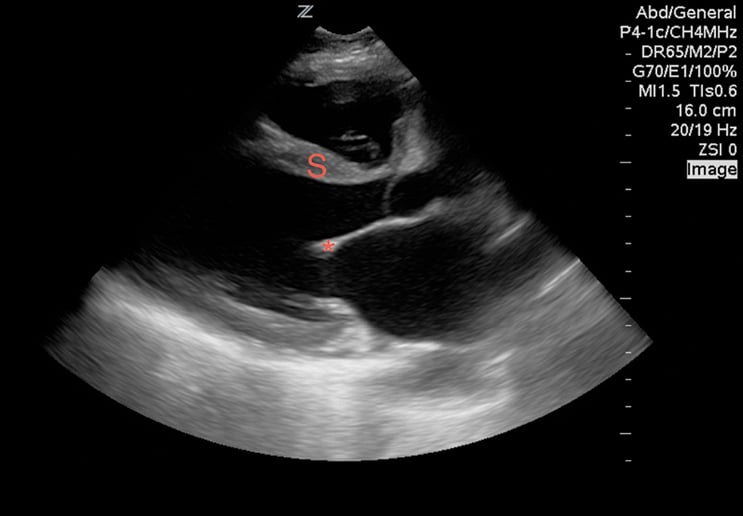
First, look at the heart. Evaluate for a pericardial effusion to rule out tamponade (Image 1). If there is an effusion, view the heart in multiple windows, including the sub-xiphoid, parasternal long, parasternal short, and the apical four-chamber views to evaluate for right ventricular collapse during diastole, which serves as sonographic evidence of tamponade.8 If you are having difficulty appreciating systole versus diastole, try using M-mode. Aim the beam over the anterior leaflet of the mitral valve in the parasternal long axis. With this image you can easily appreciate the mitral valve opening during diastole as it swings up towards the interventricular septum. The right ventricular free wall will be visible as a hyperechoic line between the outer anechoic pericardial effusion and inner anechoic blood within the right ventricle. When the mitral valve opens diastole is occurring, and you can easily appreciate right ventricular wall collapse into the right ventricle.
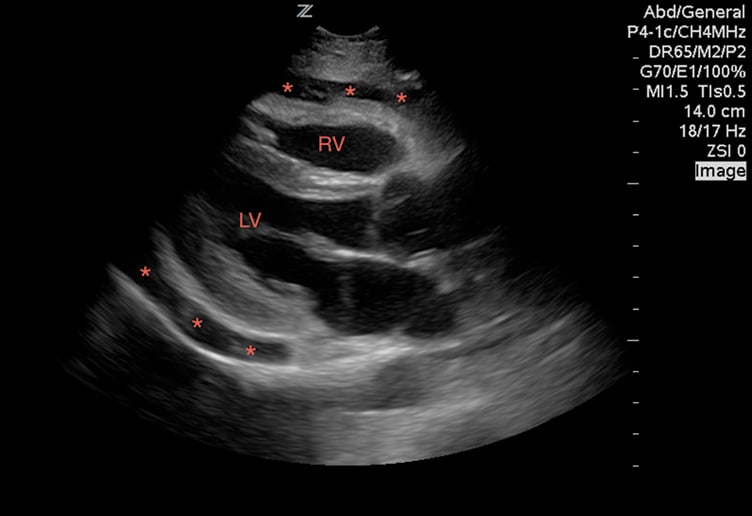
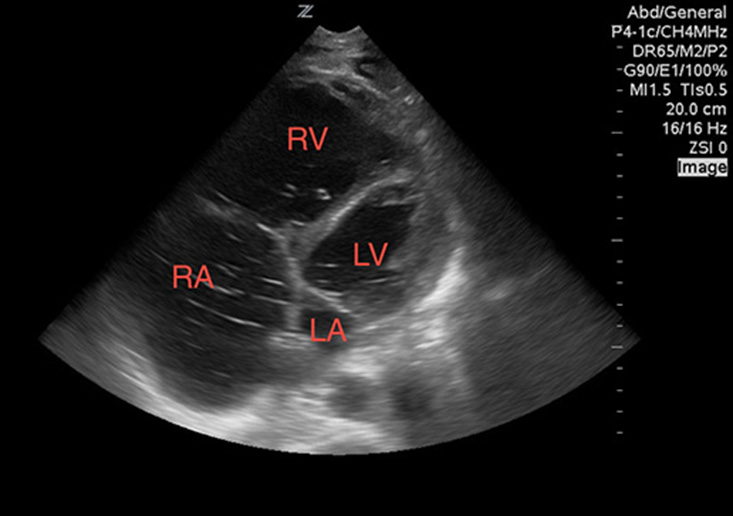
While viewing the cardiac windows, gain a sense of the overall cardiac function and ejection fraction (EF). Emergency sonographers can categorize cardiac contractility as normal, depressed, or severely depressed and can identify a severely depressed left ventricular EF with nearly 100% sensitivity.9 In a heart with a normal EF, the anterior leaflet of the mitral valve will touch, or nearly touch, the inter-ventricular septum. You may choose to measure the distance during diastole from the anterior leaflet of the mitral valve to the inter-ventricular septum using M-mode. A distance of less than 7 mm suggests a normal EF, whereas greater than 7mm is 100% sensitive for a severely depressed EF (Image 2). This is known as E-point septal separation, or EPSS.10 In the apical four-chamber view, compare the diameters of the right (RV) and left ventricle (LV). A normal RV is less than two-thirds the size of the LV. A dilated RV in the correct setting is 98% specific for right heart strain from a pulmonary embolism11 (Image 3). You may also note a wall motion abnormality as evidence of an old or new myocardial infarction. Adding color to the exam in Doppler mode enables you to also identify valve incompetence.
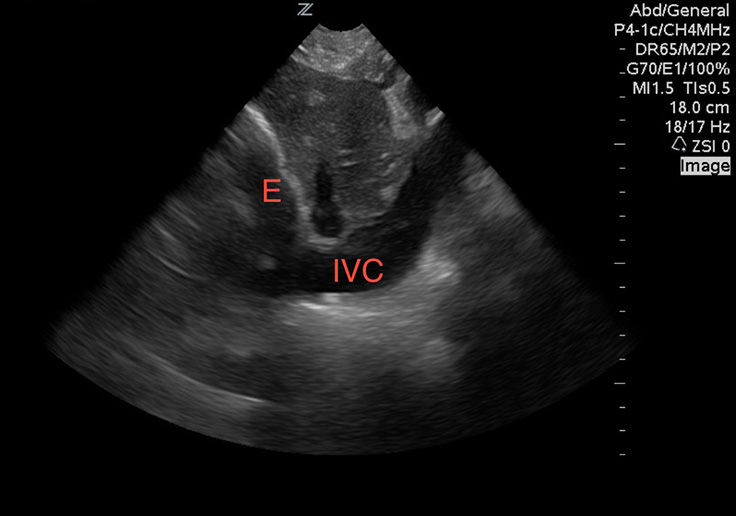
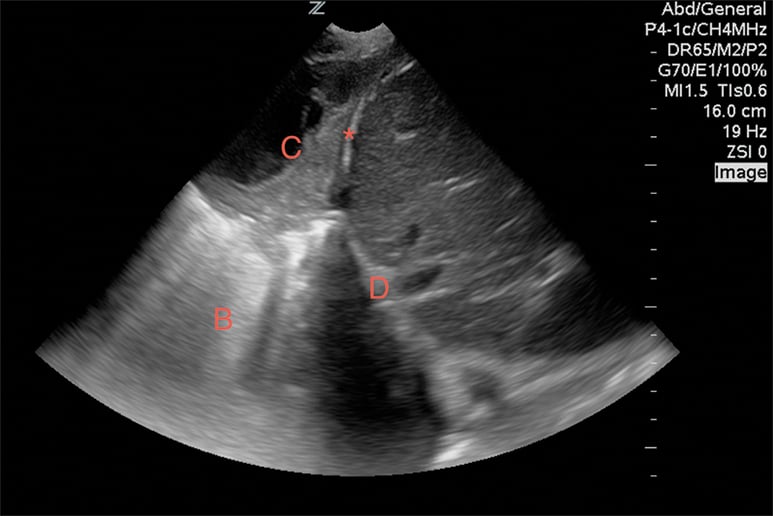
While viewing the heart in the sub-xiphoid window, align the probe indicator toward the patient”™s head and reduce the angle of the transducer to evaluate the inferior vena cava (IVC). A pericardial effusion with a full, distended IVC is very worrisome for tamponade (Image 4). If there is respiratory variability, tamponade is excluded with few exceptions. Without a pericardial effusion, a dilated IVC is consistent with fluid overloaded states. An IVC diameter that varies widely during respirations suggests an etiology other than heart failure.12
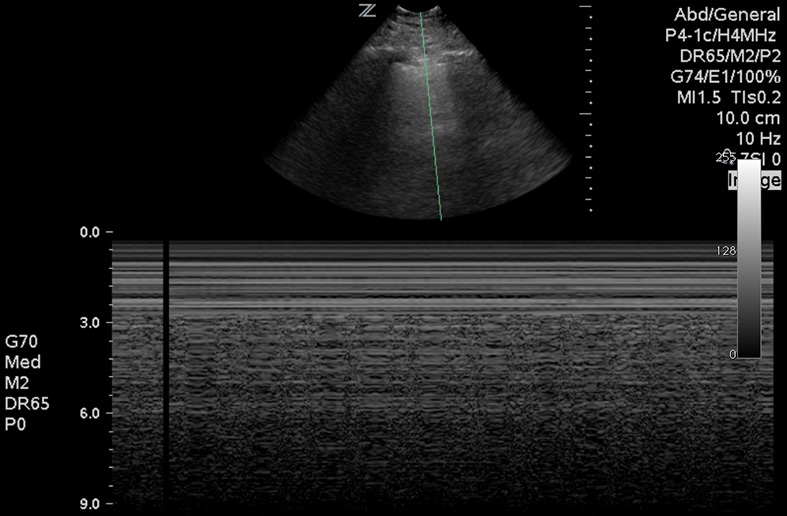
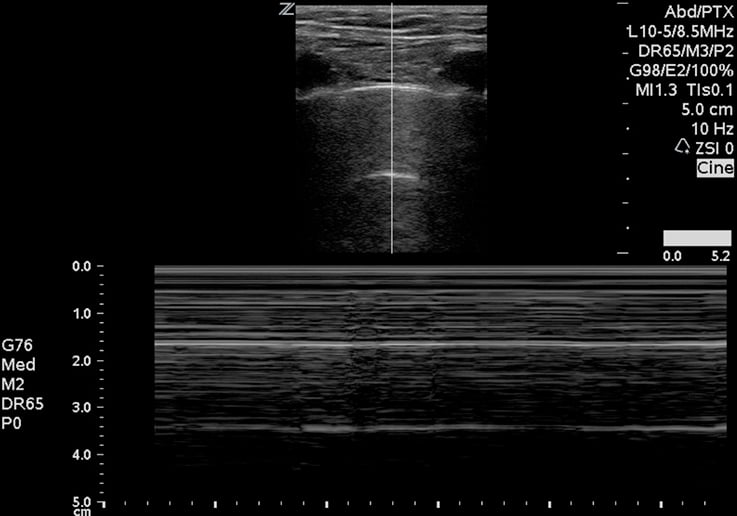
Next, move to the lungs. In a prospective study of 27 patients, lung ultrasound was more sensitive than chest X-ray and as sensitive as computed tomography in identifying pneumothoraces. Fluid in the lungs will fall to the dependent portions, whereas air rises to the superior and anterior chest. Most emergency phyisicans are familiar with the concept of lung sliding (Image 5). Start evaluating for this finding by placing the probe towards the lung apex, between the second and third intercostal space in the mid-clavicular line. Then move down through the sixth-eighth intercostal spaces. This may be done in an upright or supine position. In the setting of trauma, absent lung sliding generally means a pneumothorax is present. If clinically permitted, examination of all lung segments may reveal a lung point, which is the point of separation of the visceral and parietal pleura. This is nearly 100% specific for a pneumothorax. M-mode may also be used to evaluate for pneumothorax. The classic “seashore sign” (Image 6) is present when there is normal lung sliding, as compared to the “bar code” sign (Image 7), which is characteristic of pneumothorax. The same technique and findings are also useful to determine main stem bronchus intubation, because one lung will not be ventilated.13
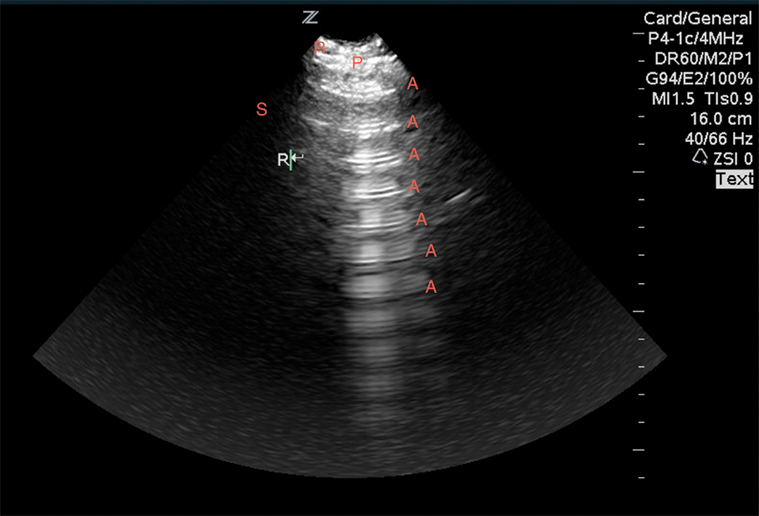
After ruling out a pneumothorax, divide each hemi-thorax into four segments bordered by the posterior axillary line, anterior axillary line, medial clavicular line and the inter-nipple line. Evaluate the lung in each quadrant for lung sliding, A-lines, B-lines, consolidations, and effusions. A-lines are regular horizontal hyperechoic lines that reverberate from the pleura and are indicative of a normal or hyperinflated lung (Image 8). If all lung segments appear to have lung sliding and A-lines, then the patient”™s differential is narrowed to that of a patient with a normal or hyperinflated chest X-ray. This may include asthma, COPD, anemia, acidosis, neuromuscular disorders, and pulmonary embolism. If pulmonary embolism is suspected, performing lower extremity deep vein thrombosis (DVT) studies can confirm (but not rule out) the diagnosis. In a prospective, 47-patient study, a two-point compression exam at the femoral and popliteal veins was nearly 100% sensitive and specific for DVT.14
A floating lung is not visualized in this image.
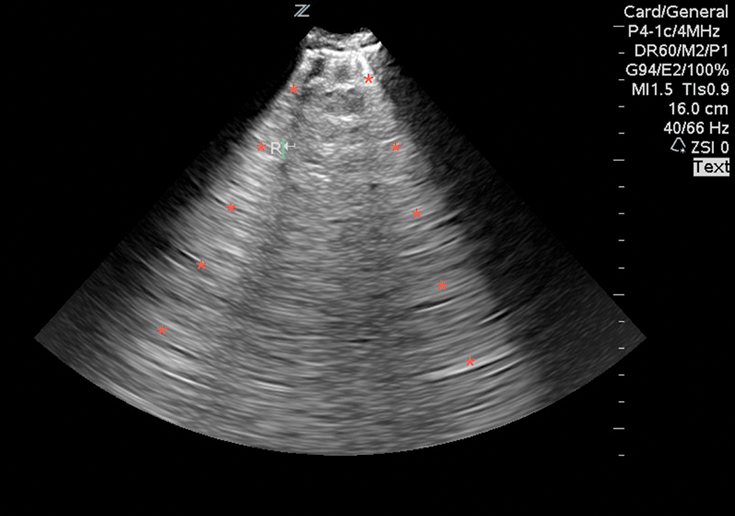
B-lines are hyperechoic vertical lines that originate from the pleural line, obliterate A-lines, and often run off the bottom of the image (Image 9). Three or more in the screen are indicative of an interstitial syndrome. A diffuse B-line pattern in all lung segments narrows the differential to those conditions that present with a diffuse interstitial lung pattern, such as pulmonary edema (cardiogenic and non-cardiogenic), multifocal pneumonia, and pulmonary fibrosis. A focal B-line pattern in one or two lung segments suggests a focal interstitial process, such as pneumonia, pulmonary contusion, or pulmonary infarction.
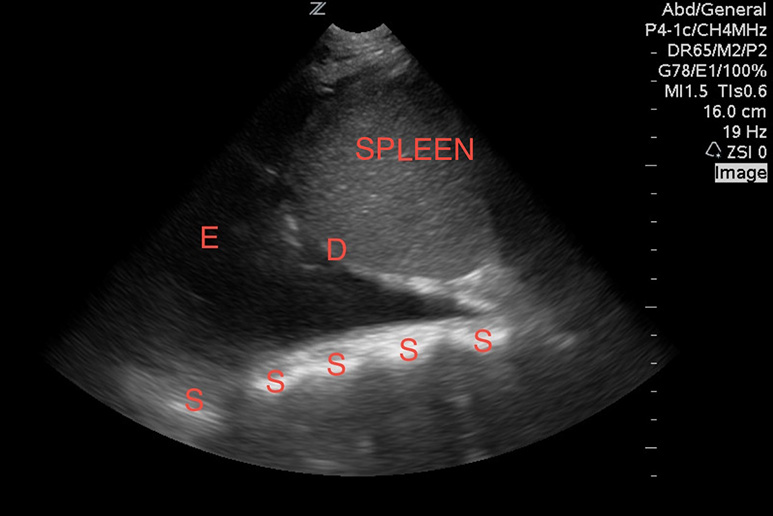
Unless loculated, pleural effusions should be found in the dependent portions of the lung, and can most easily be visualized at the lower lung segments and costophrenic angles. Without effusions, a normal mirror artifact of the liver or spleen is seen reflecting above the diaphragm, and the spine should not be visible, as air scatters the ultrasound waves. However, when a pleural effusion is present, an anechoic pocket of fluid can be seen, and often the floating free edge of the lung can be visualized (Image 10). Since ultrasound waves are transmitted well through fluid, the spine may be apparent. This is called the “spine sign.” Consolidated lung appears like solid tissue on ultrasound and is often referred to as “hepatization” of lung, as it has a similar appearance to the liver (Image 11).15
Combining the cardiac, IVC, lung, and DVT POCUS exams rapidly narrows the differential diagnosis and results in early directed management. Ultrasound may cost you more time at the bedside, but the initial investment of time is well worth it when it enables you to identify important pathology, make critical diagnoses early, and save lives. Multiple studies demonstrated the relative ease and short period of time it takes to use these techniques effectively.16 However, even expert sonographers should understand their limitations and not hesitate to employ other diagnostic tools when necessary. The greatest limitations of POCUS are the operator”™s expertise and the patient”™s body habitus.
Case resolution
On ultrasound, the patient had a markedly decreased EF, dilated IVC, diffuse B-line pattern on lung exam, and negative DVT studies. Additionally, he had bilateral moderate pleural effusions and a modest amount of abdominal free fluid. His clinical picture was consistent with congestive heart failure and he improved with BiPAP, nitroglycerin, and diuretics. He was admitted to the cardiology service and was discharged two days later on a new medication regimen.
REFERENCES
- Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest. 2011;139:1140”“1147.
- Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17:R208.
- Ding W, ShenY, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;1404:859-866.
- Cortellaro F, Colombo S, Coen D, Duca PG. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J. 2012;29:19”“23.
- Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135:1433”“1439.
- Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117”“125.
- Mantuani, D., Nagdev, A. Three-view bedside ultrasound to differentiate acute decompensated heart failure from chronic obstructive pulmonary disease. Am J Emerg Med. 2013;31(4):759-e3.
- Nagdev A, Stone M. Point-of-care ultrasound evaluation of pericardial effusions: Does the patient have cardiac tamponade? Resuscitation.2011;82:671-673
- Anderson, Kenton L, et al. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214
- McKaigney, Conor J, et al. E-point septal separation: a bedside tool for emergency physician assessment of the left ventricular ejection fraction. Am J of Emerg Med. 2014;32(6):493-497
- Dresden, Scott, et al. Right ventricular dilatation on bedside echocardiography performed by emergency physicians aids in the diagnosis of pulmonary embolism. Annals of Emerg Med. 2014;63(1):16-24.
- Anderson et al. 2013.
- Rowan, Kevin R, et al. Traumatic pneumothorax detection with thoracic US: Correlation with chest radiography and CT-initial experience. Radiology. 2002;225(1):210-214.
- Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601”“610.
- Volpicelli, Giovanni, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive care medicine. 2012;38(4):577-591.
- Noble, Vicki E., et al. Evaluation of a thoracic ultrasound training module for the detection of pneumothorax and pulmonary edema by prehospital physician care providers. BMC Med Educ. 2009;9(1):3.