Atrial fibrillation is 4-6 times more common in HCM patients than in the general population, and it carries an eightfold increase in the risk of stroke.
A 46-year-old female presents by EMS with tachycardia and hypotension. En route the patient received two rounds of adenosine with no improvement. On initial assessment, the patient is somnolent but arousable and protecting her airway. The first set of vital signs reveals a blood pressure of 70/30 and the monitor shows atrial fibrillation with a rapid ventricular rate between 160 to 180 bpm. The patient receives a 1 L bolus of normal saline without significant improvement of her heart rate or blood pressure. The decision is then made to perform a synchronized cardioversion. She is sedated with etomidate and an initial 150 J biphasic shock is delivered without any change in rhythm. A second shock at 200 J is delivered, which is again unsuccessful. Further chart review reveals that the patient has a history of hypertrophic cardiomyopathy with severe left ventricular outflow tract obstruction.
Hypertrophic Cardiomyopathy
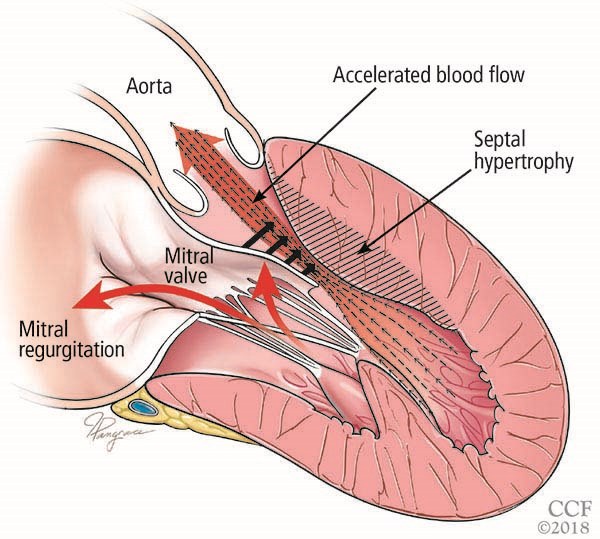
Hypertrophic cardiomyopathy (HCM) is a genetically determined autosomal dominant disorder that causes abnormal development of cardiac myocytes and intramural coronary arterioles.1 Left ventricular (LV) hypertrophy is a defining characteristic of HCM. Depending on the specific morphology, patients can develop one or more of the following: LV outflow tract (LVOT) obstruction, diastolic dysfunction, myocardial ischemia and mitral regurgitation.2 LVOT obstruction is caused by systolic anterior motion of the mitral valve against a hypertrophied septum.
Broadly, patients will present with symptoms related to heart failure, chest pain and dysrhythmias. Dyspnea, chest pain, and syncope with exertion should raise suspicion for HCM particularly in younger patients with a family history of early sudden cardiac death. Physical exam findings can be non-specific and associated with the degree of LVOT obstruction. A systolic murmur that increases with valsalva and going to an upright position from supine is suggestive of HCM.
While management of HCM can be complex and based on several factors, initial therapy in symptomatic patients is focused on reducing the LVOT obstruction and reducing myocardial oxygen demand. This is done by optimizing preload and through the use of negative inotropic agents such as beta blockers, nondihydropyridine calcium channel blockers, and disopyramide. Further down the algorithm are invasive measures such as pacemakers, automatic implantable cardioverter-defibrillators (AICDs) and septal myomectomies, which are beyond the scope of this discussion.5 Here we will focus on dysrhythmia as a complication of HCM.
Atrial Fibrillation in HCM
Atrial fibrillation (AF) is the most common dysrhythmia, both in the general population and in patients with HCM.3 However, atrial fibrillation is 4-6 times more common in HCM patients than the general population. Approximately 20% of HCM patients will go on to develop AF.3 AF is an independent predictor of all-cause mortality in HCM and carries an eightfold increase in risk of stroke.3 Barring contraindications, all HCM patients with AF should receive anticoagulation.
Patients with HCM and AF who go into rapid ventricular response are particularly high risk for hemodynamic collapse. This is in large part due to preload dependence in the heart with HCM. In the setting of atrial fibrillation, there is a loss of atrial kick, which subsequently reduces preload. In combination with a high ventricular rate, there is a decrease in cardiac output secondary to a reduction in LV filling time. This physiologic response becomes life-threatening in the setting of baseline end-diastolic dysfunction as in HCM. It is imperative to ensure that preload is optimized by giving IV fluids and if needed, phenylephrine. Positive inotropic agents such as dopamine, dobutamine and norepinephrine should be avoided. An unstable patient with HCM and AF with RVR still requires electrical cardioversion, but it may fail if preload is not addressed. If the patient is still refractory to electrical cardioversion, amiodarone can be administered. The dose will be 150 mg IV bolus followed by a continuous infusion at 1 mg/min. If at this point, the patient is still refractory, cardiology should be emergently consulted for further management.
Case Resolution
Cardiology is consulted and comes emergently to the bedside. The patient is given an additional 2 L of normal saline. A 150 mg bolus of IV amiodarone is given followed by a continuous infusion rate of 1 mg/min. Following this, a third shock is delivered at 200J which successfully converts the patient to sinus rhythm. Her blood pressure and mental status improve. She is started on a heparin drip and transferred to the cardiac intensive care unit. During her hospital course, an AICD is placed for primary prevention of sudden cardiac death. Subsequently, she undergoes a septal myectomy, mitral valve repair with papillary muscle reorientation, pulmonary vein isolation, and placement of left atrial appendage clip. She is continued on her beta blocker and anticoagulation.
Key Points
● Approximately 20% of patients with hypertrophic cardiomyopathy will have atrial fibrillation.
● HCM patients in AF with RVR are at high risk for hemodynamic collapse as a result of decreased filling time, worsened LVOT obstruction and decreased cardiac output.
● In a hemodynamically unstable HCM patient with AF, in addition to performing synchronized cardioversion, ensure that the patient has adequate preload.
● Phenylephrine can be used to augment blood pressure after fluid resuscitation, but positive inotropic drugs should be avoided.
● Amiodarone can be considered in atrial fibrillation refractory to electrical cardioversion.
Acknowledgements
Special thanks to Thomas Noeller, MD; Lauren Gustafson, MD; and Dhruv Amratia, MD, for their feedback.
Figure reprinted with permission from: Young L, Smedira NG, Tower-Rader A, Lever H, Desai MY. Hypertrophic cardiomyopathy: A complex disease. Cleve Clin J Med. 2018;85(5):399-411. Copyright ©2018 Cleveland Clinic Foundation. All rights reserved.
References
1. Maron M. Hypertrophic cardiomyopathy: Clinical manifestations, diagnosis, and evaluation. UpToDate. Last updated November 16, 2018.
2. Maron M. Hypertrophic cardiomyopathy: Morphologic variants and the pathophysiology of left ventricular outflow tract obstruction. UpToDate. Last updated December 19, 2018.
3. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy. Circulation. 2011;124(24):e783-e831.
4. Macintyre C, Lakdawala N. Management of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation. 2016;133(19):1901–1905.
5. Fifer MA. Most Fully Informed Patients Choose Septal Ablation Over Septal Myectomy. Circulation. 2007;116(2):207–216.
6. Robinson A, Kramer CM. Imaging in Hypertrophic Cardiomyopathy. American College of Cardiology.