An estimated 6.5 million Americans have heart failure (HF), with an approximate 5-year mortality at 50-55%.1-3
HF is predominantly caused by ischemia, along with valvulopathies, cardiomyopathies, toxins, infections, arrhythmias, and lung disease, leading to ventricular dysfunction.4 Ventricular dysfunction leads to decreased cardiac output and hypotension causing a neurohormonal response. This prompts increased vascular tone, increased volume retention, and ventricular remodeling.5 As preload and afterload increases it leads to a vicious cycle of increased cardiac strain causing worsening ventricular dysfunction.6
Heart failure is a broad disease and encompasses patients who have both preserved ejection fraction (EF > 50% (HFpEF)) and reduced ejection fraction ((HFrEF) EF< 40%). Acute decompensated heart failure (ADHF) may be the first presentation of the patient’s heart failure or more generally a decompensation of chronic heart failure.
Pathophysiology
ADHF is a diverse process ranging from well appearing patients with mild pulmonary edema to cardiogenic shock.4 Patients with isolated pulmonary edema may present relatively well appearing with only minimal O2 requirements, and volume overload. As decompensation worsens, left ventricular (LV) function decreases while sympathetic tone increases causing patients to present in progressive stages of extremis characterized by severe hypoxemia and associated hypertension. This subset of critically ill, hypoxemic, hypertensive heart failure patients is acute pulmonary edema (APE).
APE is caused by overactivation of the sympathetic system by an acutely decompensated heart. Left ventricular dysfunction leads to decreased blood pressure and decreased renal perfusion.7 This leads to a sympathetic surge. The increased afterload caused by peripheral vasoconstriction and cardiotoxicity by the neurohormonal response leads to further myocardial injury. Splanchnic vasoconstriction leads to a redistribution of volume in the body causing increased preload.8 This leads to both increased preload and afterload causing pulmonary edema.
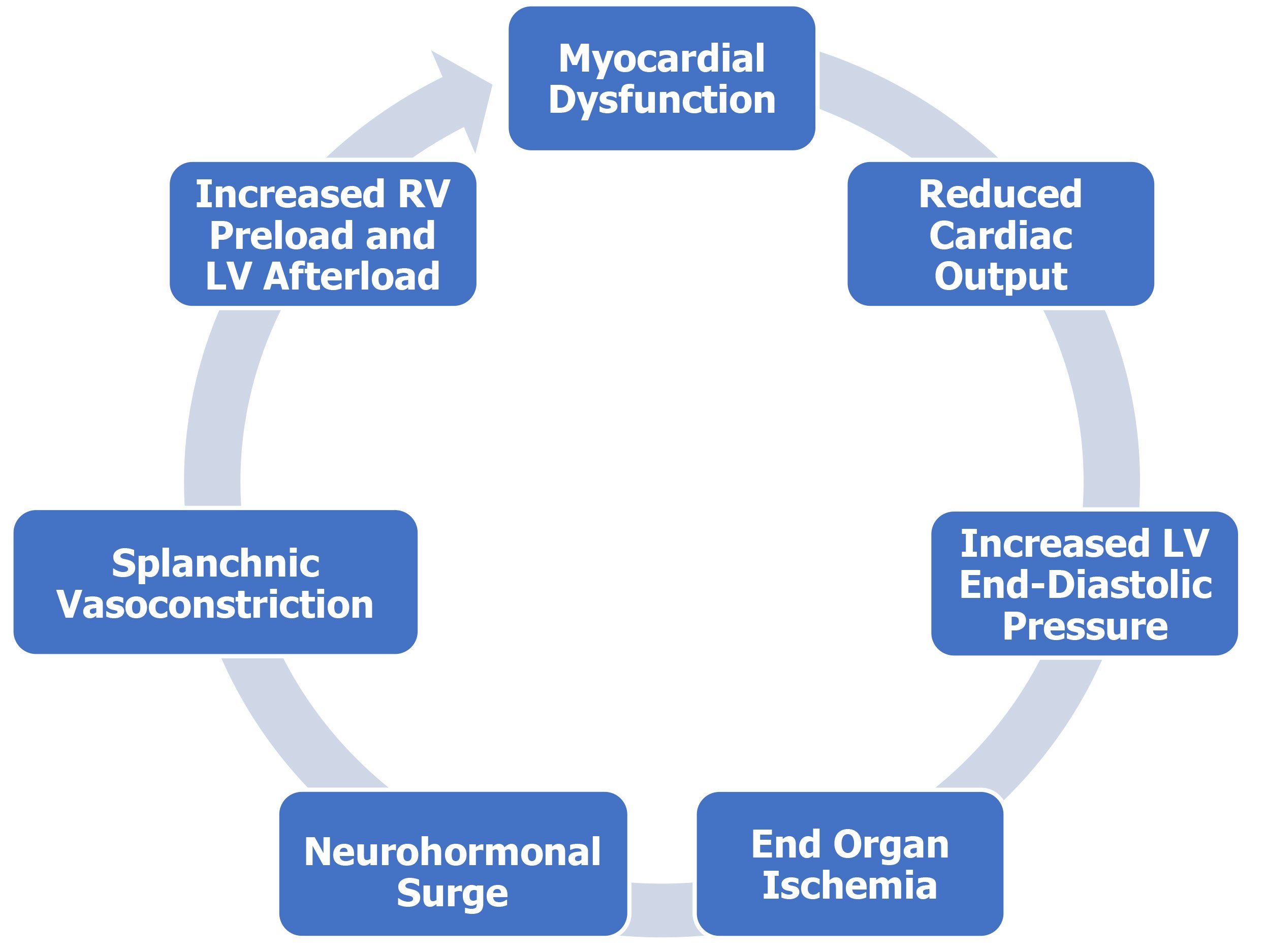
Figure 1: Acute Pulmonary Edema
Finally, at the far end of the spectrum is cardiogenic shock. As cardiac function deteriorates, these patients become hypotensive and/or exhibit evidence of tissue hypoperfusion. Symptoms of end organ dysfunction such as altered mental status, severe dyspnea, decreased urine output, and cold extremities dominate the clinical exam, while global indicators of malperfusion such as elevated serum lactate may be present.
A subset of ADHF patients maintain a normal EF including those with HFpEF, high output cardiac failure, and RV failure.
HFpEF is extremely prevalent, representing half of all cases of HF.9 HFpEF is characterized by increased LV stiffness and impaired relaxation leading to poor tolerance of tachycardia and atrial fibrillation as well as poor compensation to changes in afterload and preload.10 Despite significant differences in pathophysiology, patients with acutely decompensated HFpEF present similarly to their counterparts with reduced EF.
High output heart failure is characterized by decreased systemic vascular resistance secondary to underlying conditions such as morbid obesity, thyroid storm, cirrhosis or AV shunt resulting in a compensatory increase in ejection fraction as well as retention of salt and water. Patients with high output cardiac HF present dyspneic, volume overloaded, tachycardic but with warm, well perfused distal extremities.11,12
Finally, right ventricular failure can occur as a consequence of chronic pulmonary hypertension secondary to a variety of etiologies including chronic pulmonary disease and HFpEF or can occur abruptly secondary to acute processes such as pulmonary embolism or right-sided acute myocardial infarction. Furthermore, RV failure can exist in isolation or concomitant to LV failure. Ultimately, while RV failure and HOHF are an important etiology of ADHF, their diagnosis and management are sufficiently distinct to deserve its own discussion.
While ADHF encompasses a broad group of patients there are four hemodynamic subsets that provides a useful framework. Recognition of volume overload, pump failure and shock remain an important aspect of the management of patients with ADHF.13
| Congestion (-) | Congestion (+) | |
| Hypoperfusion (-) | Warm-Dry | Warm-Wet |
| Hypoperfusion (+) | Cold-Dry | Cold-Wet |
Diagnosis
The challenge in resuscitating the undifferentiated respiratory failure patient begins with the correct identification of the etiology. Significant clinical overlap exists between a variety of common etiologies of hypoxemia while the presence of concomitant processes is not uncommon. The rapid and accurate diagnosis of ADHF is critical.
History and Physical Exam: The diagnosis of ADHF with undifferentiated respiratory failure starts with a focused H&P. A PMH of heart failure, myocardial infarction or coronary artery disease with symptoms such as dyspnea (most common), orthopnea and peripheral edema suggest HF as a potential etiology.14 Physical exam findings such as a 3rd heart sound, JVD, or hepatojugular reflux are highly specific but insensitive. While crackles on auscultation are present in 60-70% of HF patients, this finding suffers from mediocre sensitivity and specificity.15 Overall, while the H&P play an important role in diagnosis, no finding is adequate to fully diagnosis HF.16
|
Finding |
Positive LR |
Negative LR |
|
Heart Failure/ MI/ CAD |
4.4/5.8/3.1 |
.45/.69/.68 |
|
Paroxysmal Nocturnal Dyspnea/orthopnea/edema |
2.6/2.2/2.1 |
.7/.65/.64 |
|
Third Heart Sound/ Abdominojugular reflex/ JVD |
11/6.4/5.1 |
.88/.79/.66 |
Chest radiography: A chest X-rayis essential with pulmonary venous congestion, hilar redistribution, pleural effusions, and interstitial edema consistent with ADHF.4 Additionally, CXR will identify non-cardiac precipitants of respiratory failure such as pneumonia or pneumothorax. However, while chest radiography findings are specific for HF they suffer from poor sensitivity, with 20% of ADHF having a negative CXR.15-17
|
Finding on CXR |
Positive LR |
Negative LR |
|
Venous congestion |
12 |
.48 |
|
Interstitial edema |
12 |
.68 |
|
Alveolar edema |
6 |
.95 |
|
Cardiomegaly |
3.3 |
.33 |
|
Pleural effusion |
3.2 |
.81 |
Ultrasound: Several ultrasound modalities are useful in the diagnosis of ADHF. The first modality is the qualitative assessment of left ventricular function. The second modality is the assessment of the intra-abdominal segment of the IVC just distal to the right atrium. In patients with elevated right heart pressures in the setting of ADHF, the IVC will often be dilated > 2 cm with minimal to no collapse during respirations. The third modality is lung ultrasound examining both hemithoraces for evidence of subpleural edema in the form of B-lines.18 While each finding is useful individually, Anderson et al. investigated a “triple scan” protocol utilizing all 3 modalities in patients presenting to the ED with ADHF as a part of their differential.19 The study found that LVEF <45%, IVC-Collapsibility Index <20%, and >10 B-lines of US was 100% specific for ADHF. Even without the inclusion of lung US, LVEF plus IVC alone was 98% specific. Unfortunately, both groups were associated with relatively poor sensitivity, 36% and 48% respectively.19
However, given the prevalence of etiologies of ADHF with normal EF such as HFpEF and RV failure, this is not surprising. Supplementing your ultrasound with modalities to evaluate for RV and diastolic dysfunction may provide additional value in ruling out ADHF. In contrast to a CXR, a complete evaluation of the heart, IVC, and lungs can reliably be performed quickly, providing critical information to guide management immediately.20-22 In a prospective observational study, EM residents were able to more accurately diagnose pulmonary edema with lung US than by chest x-ray.23 Another RCT supported these findings by showing that using POCUS alongside standard diagnostic modalities improves the rate of achieving the correct diagnosis within a shorter period of time than standard diagnostic modalities alone.24
Biomarkers: Biomarkers such as brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) are highly sensitive for ADHF and provide useful information in the initial evaluation of the ED patient with acute respiratory failure.25 The “Breathing Not Properly” trial enrolled ED patients presenting with acute dyspnea and found that a BNP cutoff of < 100 pg/mL had a sensitivity of 90% with a negative predictive value of 89%. Decreasing the cutoff to 50 pg/mL increased the sensitivity to 97% and NPV to 96%.26 However, while a BNP >500 pg/mL makes the diagnosis of ADHF likely, serum levels of BNP can be increased by a variety of factors; as such the specificity of the test is limited.26 Similarly, the PRIDE study investigated the utility of NT-proBNP and found that a cutoff value of <300 pg/mL was associated with both a sensitivity and NPV of 99%.27 In a subsequent pooled analysis , age adjusted cutoffs improved the accuracy of NT-proBNP with values of <450 pg/mL in age < 50 years, < 900 for age 50-75, and < 1800 for age >75 as optimal cut-points suggested for confirmatory testing.28 Be careful though, as BNP can be elevated in other disease states such as pulmonary embolism.29 Use this in conjunction with clinical gestalt.
Electrocardiogram: While abnormal findings on ECG are non-specific for the diagnosis of ADHF, a prompt ECG is crucial in establishing precipitants of failure such as acute myocardial infarction, active ischemia, tachyarrhythmias, and bradyarrhythmias and thus should be obtained promptly. While abnormal ECG is not useful for confirming diagnosis, a normal ECG may have additional value in ruling out the possibility of ADHF.30
Labs: A BMP, CBC, troponin, LFTs and TSH should be obtained for patients with new onset ADHF.4 D-dimer should be obtained if there is a concern for PE. Hyponatremia in patients with ADHF is usually dilutional and indicates poor clinical outcomes.31,32
Overall Approach: The evaluation of the patient presenting with suspicion of ADHF is multi-modal including focused history and physical, POCUS, CXR, EKG and biomarkers. Evidence of reduced LVEF, non-respirophasic IVC with or without bilateral B-lines on POCUS essentially confirms the diagnosis of ADHF. CXR reinforces findings on POCUS and rules out coexisting pathology. ECG provides rapid diagnosis of potential precipitants of failure. If radiographic and ultrasound findings are equivocal, BNP or NT-proBNP levels < 100 or 300 pg/mL respectively, rule out the possibility of ADHF as the etiology of the patient’s respiratory failure.
Management
The patient who arrives normotensive with a gradual, indolent course of symptoms is generally not in extremis. They are managed with IV loop diuretics with consideration of low-dose vasodilators.4 We will focus on ADHF patients in extremis; those in acute pulmonary edema and cardiogenic shock.
Acute Pulmonary Edema
These patients present hypertensive with acute severe respiratory distress, hypoxemia, and b-lines that can extend to the clavicles. They require quick management to properly stabilize them.
Non-Invasive Positive Pressure Ventilation: The sickest ADHF patients require positive pressure ventilation. Endotracheal intubation carries its risks however. Thankfully, non-invasive positive pressure ventilation (NIPPV) reduces intubations, mortalities, and ICU stays for cardiogenic pulmonary edema. One Cochrane Review by Vital et al. showed that the NNT to prevent a mortality is thirteen while the NNT to prevent an intubation is 8.33 Another Cochrane Review by Collins et al. showed that early application of NIPPV reduces the relative risk of mortality by 39% and the relative risk of intubation by 57% in ED patients.34 Both CPAP and BIPAP are appropriate choices as there seems to be no difference in clinical outcomes between the two.35,36 NIPPV leads to a decrease in RV preload and increased RV afterload thus leading to decreased pulmonary congestion.37 The LV experiences both reduced preload and afterload thus improving cardiac output. In the ADHF patient in which lung compliance is impaired, NIPPV helps to recruit collapsed alveoli, reverse atelectasis, and drive fluid out of the lungs.37 These effects improve shunting and thus oxygenation. Further, NIPPV reduces overall work of breathing by assisting fatiguing respiratory muscles.
Vasodilation: To reverse the flash pulmonary edema in hypertensive cardiogenic pulmonary edema, you have to break the intense vasoconstriction causing volume redistribution and end organ ischemia by aggressively reducing the preload and afterload.This is primarily done by nitrate therapy. Nitroglycerin at low doses <100 mcg/min causes venodilation leading to pre-load reduction, while high doses leads to arterial dilation reducing afterload. High dose nitroglycerin is associated with better outcomes than with low dose nitro.38 Further, the patients in one study received boluses of nitroglycerin of up to 2 mg at a time, which is much higher than many providers routinely give, indicating that it is a safe therapy. One strategy to dose nitro is to administer a loading dose of 400 mcg/min for 2 minutes and titrate a drip from there. Alternatively, in the absence of IV access nitroglycerin can be dosed by sublingual tablet or spray.
Cardiogenic Shock
ADHF patients who are hypotensive and hypoperfusing are in cardiogenic shock. The two goals are to optimize cardiac output and increase end organ perfusion. Pharmacologically this is achieved by using both an inotrope such as dobutamine or epinephrine and a vasopressor such as norepinephrine.1 A multicenter RCT looking at dopamine vs norepinephrine in shock showed no significant difference in mortality in patients, but dopamine had an increased number of arrhythmogenic events.39 Further, a sub-group analysis showed that dopamine had an increased mortality compared to norepinephrine in patients with cardiogenic shock. In patients with refractory cardiogenic shock you can add either dobutamine or epinephrine for increased inotropic stimulation.
One trial examining dobutamine plus norepinephrine vs epinephrine in patients with dopamine-resistant cardiogenic shock showed that the two groups are similar in regard to global hemodynamic function.40 However, the epinephrine group was shown to have increased rates of transient lactic acidosis, tachyarrhythmias, and decreased gastric perfusion.
Another small randomized trial showed that epinephrine is associated with adverse cardiac, and renal effects along with increased mortality compared to dobutamine plus norepinephrine.41
A randomized trial looking at norepinephrine vs epinephrine in cardiogenic shock caused by acute MI showed that epinephrine had a higher incidence of refractory shock with similar effects on arterial pressure and cardiac index.42 Without a large RCT to definitively examine epinephrine vs dobutamine plus norepinephrine, it is most likely safer to use dobutamine plus norepinephrine.
To optimize management, an arterial line should be considered for invasive monitoring. ACS must be ruled out, as an MI would require urgent revascularization. Mechanical circulatory support systems such as ventricular assist devices (VAD), intra-aortic balloon pump, or extracorporeal membrane oxygenation (ECMO) can be considered in the setting of refractory shock as a bridge to potential heart transplant, long-term VAD therapy, or recovery.43,44
Controversies
Diuresis: Furosemide is considered standard of care in the management of ADHF. However, there is conflicting data regarding optimal timing of diuresis. A recent observational study, REALITY-AHF, concluded that early treatment with loop diuretics in patients with evidence of volume overload lead to improved hospital mortality.45 A subsequent observational study by Park et al. found that early versus delayed diuretic administration had no significant effect on mortality, with early diuresis instead being associated with increased need for mechanical ventilation.46 ADHF is not a homogenous disease, and pulmonary congestion may be the result of redistribution of fluid and not all patients with ADHF are total body volume overloaded. Overall, the decision of if and when to administer furosemide should be tailored to the individual patient, with priority given to those patients with evidence of volume overload on clinical or ultrasound examination.
The DOSE trial demonstrated that high-dose IV furosemide (2.5 times home oral dose) did not improve mortality but did lead to greater relief of dyspnea, weight change, and fluid loss compared to low-dose furosemide (home oral dose) with the consequence of only transient worsening in renal function.47 However, caution should be used regarding total dose of diuretic administered. Analysis of the ADHERE registry found that total dosage of furosemide in excess of 160 mg over the first 24 hours of hospitalization was associated with increased hospital length of stay and mortality.48
Opiates: Historically it was taught that morphine reduces air hunger and anxiety and has positive hemodynamic effects in patients with ADHF. This practice was challenged by post-hoc analysis of the ADHERE registry, which found that the administration of morphine was associated with higher rates of intubation, increased rates of ICU admissions, and greater risk of mortality.49 However, a subsequent trial by Lakobishvili et al. shows that morphine was associated with in-hospital mortality in the multivariable analysis but not in the propensity score analysis.50 This was followed by a study examining the EAHFE registry, which showed in a propensity score analysis that there was a non-significant trend toward mortality when morphine was given to treat for ADHF.51 Finally, the AHA states that morphine should only be used for palliative use in end-stage HF.52
Conclusion
Heart failure is a deadly disease that requires an understanding of the pathophysiology and diagnostic modalities to properly treat. Most HF patients will require diuresis; however, the sickest HF patients will require aggressive, focused care.
Vital signs should be immediately obtained with a focused history and physical. This should be followed immediately by POCUS, specifically looking at the lungs, heart, and IVC. This will allow you to differentiate if the patient is in cardiogenic shock (poor cardiac squeeze, cold, hypoperfusing) or acute pulmonary edema (hypertensive, B-lines up to their clavicle, and dilated IVC). Both patients can benefit from NIPPV, but how to treat their underlying condition is vastly different. APE patients require aggressive vasodilation while cardiogenic shock patients require inotropic and vasopressor support. These are some of the sickest patients to appear in the ED and require aggressive, quick treatment to turn them around.
References
1. Heidenreich PA, Albert NM, Allen LA, et al. for the American Heart Association Advocacy Coordinating Committee. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619.
2. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016–1022.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146-e603.
4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27): 2129-2200.
5a. Chung P, Hermann L. Acute Decompensated Heart Failure: Formulating an Evidence Based Approach to Diagnosis and treatment (part I). Mt Sinai J Med. 2006;73(2):506-515.
5b. Buccelletti F, Hermann L. Acute Decompensated Heart Failure: Formulating an Evidence Based Approach to Diagnosis and treatment (part II). Mt Sinai J Med. 2006;73(2):516-527.
6. Kemp, CD, Conte JV. The Pathophysiology of Heart Failure. Cardiovasc Pathol. 2012;21(5):365-371.
7. Agrawal N, Kumar A, Aggarwal P, Jamshed N. Sympathetic crashing acute pulmonary edema. Indian J. Crit Care Med. 2016;20(12):719-723.
8. Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4(5):669-675.
9. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2018;32:670-679.
10. Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High Prevalence of Occult Heart Failure With Preserved Ejection Fraction Among Patients With Atrial Fibrillation and Dyspnea. Circulation. 2018;137:534-535.
11. Mehta PA, Dubrey SW. High output heart failure. QJM. 2008;102(4):235–241.
12. Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-output heart failure: a 15-year experience. J Am Coll Cardiol. 2016;68(5):473-482.
13. Forrester JS, Diamond G, Chatterjee K, Swan HJC. Medical Therapy of Acute Myocardial Infarction by Application of Hemodynamic Subsets. 1976;295(24):1356-1362.
14. Adams JR KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: (ADHERE). Am Heart J. 2005;149(2):209-216.
15. Allen CJ, Guha K, Sharma R. How to Improve Time to Diagnosis in Acute Heart Failure – Clinical Signs and Chest X-ray. Card Fail Rev. 2015;1(2):69-74.
16. Wang CS, FitzGerald JM, Schulzer M, et al. Does This Dyspneic Patient in the Emergency Department Have Congestive Heart Failure? JAMA. 2018;294(15):1944-1956.
17. Collins SP, Lindsell CJ, Storrow AB, Abraham WT. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13-18.
18. Martindale JL. Resolution of sonographic B-lines as a measure of pulmonary decongestion in acute heart failure. Am J Emerg Med. 2016;34(6):1129-1132.
19. Anderson KL, Jeng KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214.
20. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest. 2008;134(1):117-1125.
21. Andersen GN, Haugen BO, Graven T, Salvesen O, Mjolstad OC, Dalen H. Feasibility and reliability of point-of-care pocket-sized echocardiography. Eur J Echocardiogr. 2011;12(9):665-670.
22. Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound. 2006;4:34.
23. Martindale JL, Noble VE, Liteplo A. Diagnosing pulmonary edema: lung ultrasound versus chest radiography. Eur J Emerg Med. 2013;20(5):356-360.
24. Laursen CB, Sloth E, Lassen AT, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomized controlled trial. Lancet Respir Med. 2014;2(8):638-646.
25. Silvers SM, Howell JM, Kosowsky JM, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes. Ann Emerg Med. 2007;49(5):627-669.
26. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. N Engl J Med. 2002; 347:161-167.
27. Januzzi Jr. JL, Camargo CA, Anwaruddin S, et al. The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954.
28. Januzzi JL, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2018;27(3):330-337.
29. Long B, Koyfman A, Chin EJ. Misconceptions in acute heart failure diagnosis and Management in the Emergency Department. Am J Emerg Med. 2018;36(9):1666-1673.
30. Mant J, Doust J, Roalfe A, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. 2009;13(32):1-207.
31a. Verbrugge FH, Steels P, Grieten L, et al. Hyponatremia in Acute Decompensated Heart Failure: Depletion versus Dilution. J Am Coll Cardiol. 2015;65(5):480-492.
31b.Baliga R.Hyponatremia in Acute Decompensated Heart Failure: Depletion Versus Dilution: 10 Points to Remember. J Am Coll Cardiol. 2015;65:480-492.
32. Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol. 2005;95(9A):2B-7B.
33. Vital FMR, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2013;5:CD005351.
34. Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med. 2006;48(3):260-269.
35. Li H, Hu C, Xia J, Li X, Wei H, Zeng X, Jing X. A comparison of bilevel and continuous positive airway pressure noninvasive ventilation in acute cardiogenic pulmonary edema. Am J Emerg Med. 2013;31(9):1322-1327.
36. Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care. 2006;10(2):R49.
37. Kato T, Suda S, Kasai T. Positive airway pressure therapy for heart failure. World J Cardiol. 2014;6(11):1175-1191.
38. Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: A feasibility and outcome analysis. Ann Emerg Med. 2007;50(2):144-152.
39. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789.
40. Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. Crit Care Med. 2011;39(3):450-455.
41. Tarvasmaki T, Lassus J, Varpula M, et al. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20(1):208.
42. Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173-182.
43. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-1296.
44. Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation. 2012;125(10):1304–1315.
45. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017;69(25):3042-3051.
46. Park JJ, Kim SH, Oh IY, et al. The Effect of Door-to-Diuretic Time on Clinical Outcomes in Patients With Acute Heart Failure. JACC Heart Fail. 2018;6(4):286-294.
47. Felker GM, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797-805.
48. Peacock WF, Costanzo MR, De Marco T, et al. Impact of Intravenous Loop Diuretics on Outcomes of Patients Hospitalized with Acute Decompensated Heart Failure: Insights from the ADHERE Registry. Cardiology. 2009;113(1):12-19.
49. Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and Outcomes in Acute Decompensated Heart Failure: An ADHERE Analysis. Emerg Med J. 2008;25(4):205-209.
50. Iakobishvili Z, Cohen E, Garty M, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13(2):76-80.
51. Miró Ò, Gil V, Martín-Sánchez FJ, et al. Morphine use in the emergency department and outcomes of patients with acute heart failure: A propensity score-matching analysis based on the EAHFE Registry. Chest. 2017;152(4):821-832.
52. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;134(13):2129-200.