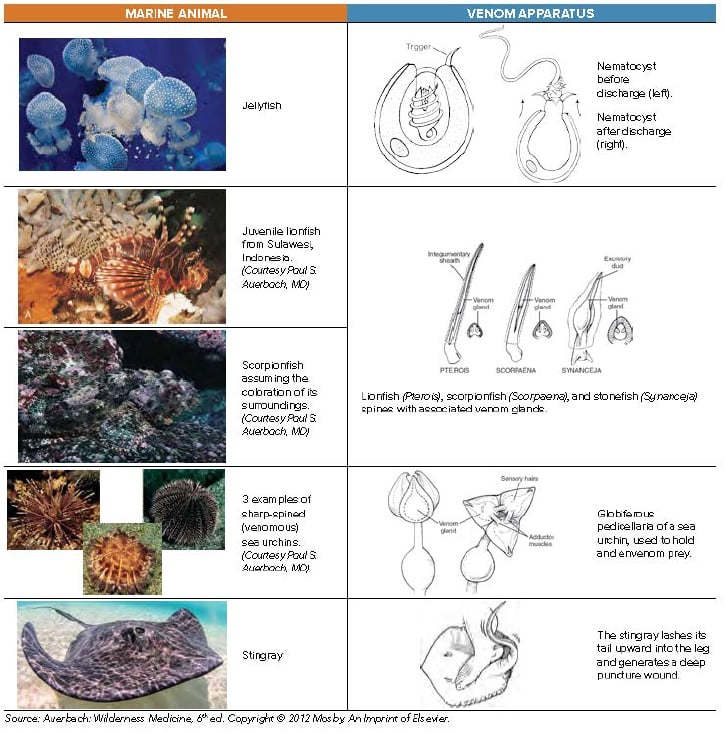
When medical toxicologist Matthew Sztajnkrycer, MD, FACEP, was a second-year resident, he answered a truly strange telemetry call. EMS had arrived on scene of a minor motor vehicle accident to find a young woman screaming in pain in the front seat. She had been transporting a lionfish to a new aquarium for its grand opening and had placed the container between her thighs to protect the precious cargo. The container had shattered, resulting in multiple stings on her thighs. EMS had called to ask if they should urinate on the patient's legs to neutralize the venom.
Far away from the natural habitat of lionfish, Dr. Sztajnkrycer was expected to provide advice on how to care for an acute marine envenomation. (Spoiler alert: He did not recommend that the EMS providers relieve themselves on the patient.) This article focuses on the treatment of the most common perpetrators of marine envenomations. While there are hundreds of unique venomous fish species, 5 simple steps will help you treat most patients presenting with acute marine envenomation.
1. Quickly immerse the wound or sting in hot water.
This seemingly simple first step is one of the most important. Hot water immersion (HWI) has been shown to significantly decrease the pain associated with envenomation.1,2 Additionally, it has been shown to deactivate heat labile proteins and enzymes, thereby minimizing any systemic effects.3 The victim's affected extremity should be immersed in hot water (42 °-45 °C) as tolerated for 30 to 90 minutes or until there is significant pain relief. Immersion can then be repeated as needed for pain control.4 If using a basin, it is important to maintain water temperature via continuous monitoring and add additional hot water as needed. Of course, the temperature should not exceed 45 °C due to the risk of thermal burns. If available, showering is preferable due to improved ability to wash off any remaining stinging cells, adjust the temperature quickly, and maintain a constant temperature.
While HWI is the most important first step in any painful marine envenomation, jellyfish deserve special consideration. Before HWI, tentacles should be washed off with seawater, as fresh water may induce nematocyst discharge from the tentacles and worsen the patient's envenomation. Secondly, if the patient is stung in areas known to have Carukia barnesi (causes Irukandji syndrome, Northern Australia), Carybdea alata (box jellyfish species specific to Hawaii), or Pelagia noctiluca (Mediterranean), consider immersing the stung area in vinegar for at least 30 seconds to inactivate any remaining undischarged nematocysts on the patient's skin.5 While vinegar is effective in these species, it can actually cause nematocyst discharge in others.6 If in doubt, lidocaine solution may be a good alternative, as it not only provides local anesthesia but also hinders nematocyst discharge via interactions with calcium ion channels.5
2. Provide additional pain control as needed.
If repeated HWI provides inadequate pain control, a regional nerve block or local anesthetic with bupivacaine or lidocaine may be necessary.7 Remember that the maximal allowable single dose of bupivacaine 0.25% is 3mg/kg, or 70 mL per average adult. Lidocaine's maximal allowable single dose is 4.5 mg/kg of 1% without epinephrine, or 30 mL per average adult. Some patients may require additional parenteral narcotics. Many historic remedies have not been shown to work, including meat tenderizer, magnesium sulfate, local injection of potassium permanganate, congo red, alcohol, and – of course – urine.8 These treatments are anecdotal and may even contribute to further tissue damage.
3. Thoroughly irrigate and explore wounds, remove spines and debride necrotic tissue.
Removal of spines limits envenomation, thus limiting systemic effects and reducing pain. Control of systemic effects becomes especially important in cases of multiple barbs or spines, such as in sea urchin envenomations. Retained spines increase risk of polymicrobial infections, leading to prolonged and complicated wound healing.9 Finally, retained spines may cause significant long-term morbidity, especially if located near a joint. Sea urchin spines induce severe synovitis, which can progress to arthritis over time.1
When removing easily accessible spines, grasp gently to avoid crushing the spines. Sea urchins in particular have fragile spines that are easily fractured. Spines located near joints should be surgically removed, even if they are easily grasped. In the case of interphalangeal joint involvement, the affected finger should be splinted until the spine is surgically removed to limit fragmentation and further penetration into the joint space. To remove stinging units that are invisible on the skin, such as jellyfish nematocysts or sea urchin pedicullariae, apply shaving cream and remove with a sharp edge, like a razor or a credit card. If shaving cream is not available, use a paste of baking soda or flour, or a slurry of sand and seawater.10
If you suspect that your patient has a retained spine, start with a radiograph. Plain film imaging may show radiopaque fragments of spines or barbs. Unfortunately, radiographs will not reveal hypodense fragments of integumentary and glandular tissues, in which venom may be concentrated. MRIs may help, but they are not cost effective for routine evaluation of all wounds. One retrospective study found that out of 100 victims of stingray envenomations presenting to the emergency room, radiographs were ordered on 59 patients. All radiographs were negative for foreign bodies, and the patients did not develop complications suggestive of missed retained barbs.11 While this may suggest that radiographs are unhelpful, there have been case reports highlighting a significant potential for reduction of morbidity by identifying the rare barb or spine on a radiograph. For example, take the 44-year-old male who sustained a stingray whip injury to his dominant wrist but did not receive radiographs on initial presentation. One month later he re-presented with swelling of his hand and inability to extend his third and fourth digits. A plain x-ray of his wrist at this time revealed two foreign bodies resembling spines, and surgical exploration revealed copious purulent material and extensive tendon injury. The patient underwent prolonged physical and occupational therapy, regaining only adequate – but not full – function of his hand.12
The very rare exception to the rule of removing spines is when victims present with stingray impalement injuries to the chest, abdomen, or neck. Treat these injuries exactly as you would any other impalement injury: Secure the spine to limit movement until the patient can be brought to the operating room for exploration and removal.
4. Prescribe antibiotics when appropriate.
Prophylactic antibiotics are recommended for injuries with significant infectious potential, such as large lacerations, significantly contaminated wounds, suspected retained organic foreign material, deep puncture wounds (especially of the hands or feet), and in immunosuppressed patients. The chosen antibiotic should cover Staphylococcus and Streptococcus, as well as Vibrio species. Ciprofloxacin, doxycycline, or trimethoprim-sulfamethoxazole are appropriate options.13
Patients who present with rapidly progressive cellulitis or myositis must be covered for Vibrio vulnificus or Vibrio parahaemolyticus. Options include parenteral meropenem, an aminoglycoside, ciprofloxacin, or ceftazidime in combination with tetracycline.14
5. Observe for onset of systemic symptoms for at least 3-4 hours.
If the disposition plan is to treat and discharge home, patients should be observed for at least 3-4 hours for systemic symptoms. If symptoms such as severe muscle cramping, respiratory distress, hypotension, or arrhythmias develop, the patient warrants admission for supportive care. Marine antivenoms currently only exist for 4 species: Chironex fleckeri (Australian box jellyfish), Synanceja trachynis (stonefish), Enhydrina schistose (beaked sea snake), and Notechis scutatus (tiger snake).*
Conclusion
These 5 steps will help emergency physicians initiate treatment for marine envenomations, even when the offending species is unknown. Of course, assessing airway, breathing, and circulation is always the primary consideration, and cases of potential systemic envenomation are no exception. Remember to reach out to your local poison control center for additional guidance.
*Sea snake bites are treated differently than other marine envenomations and thus are outside the scope of this article. In general, sea snake envenomations are treated similarly to their terrestrial counterparts.
References
- Conrad L, Stokes B, Worsley D, et al. A randomized controlled trial of hot water (45 °C) immersion versus ice packs for pain relief in bluebottle stings. Med J Aust. 2006;184:329-333.
- Muirhead D. Applying pain theory in fish spine envenomation. South Pacific Underwater Med Soc J. 2002;32:150–3.
- Carrette TJ, Cullen P, Little M, et al. Temperature effects on box jellyfish venom: a possible treatment for envenomed patients? Med J Aust. 2002;177:654–5.
- Auerbach, PS. Envenomation by aquatic vertebrates. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, PA: Mosby, Inc; 2012:1628-1645.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:421-429.
- Fenner PA, Williamson JA, Burnett JW, et al. First aid treatment of jellyfish stings in Australia: response to a newly differentiated species. Med J Aust. 1993;158:498-501.
- Garyfallou GT, Madden JF. Lionfish envenomation. Ann Emerg Med. 1996;28(4):456-457.
- Kizer KW, McKinnery HE, Auerbach PS. Scorpaenidae envenomation: a five-year poison center experience. JAMA. 1985;253(6)807-810.
- Bendt RR, Auerbach PS. Foreign body reaction following stingray envenomation. J Wilderness Med. 1991;2:298-303.
- Auerbach, PS. Envenomation by aquatic invertebrates. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, PA: Mosby, Inc; 2012:1596-1628.
- Clark RF, Girard RH, Rao D, et al. Stingray envenomation: a retrospective review of clinical presentation and treatment in 119 cases. J Emerg Med. 2007;33(1):33-37.
- O'Malley GF, O'Malley RN, Pham O, et al. Retained stingray barb and the importance of imaging. Wilderness Environ Med. 2015;26(3):375-379.
- Isbister GK. Venomous fish stings in tropical northern Australia. Am J Emerg Med. 2001; 19(7):561-565.
- Auerbach PS. Injuries from nonvenomous aquatic animals. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, PA: Mosby, Inc; 2012:1562-1596.



