It’s 2 am in a busy Level I trauma center. The triage nurse brings back a 30-year-old female and states, “I think she is having a panic attack.” The patient tells you she was awakened by her infant twins and noticed a Charley horse in the right side of her neck. She then developed substernal chest pain that was severe, constant, and radiated to her back. En route to the ED, she developed left upper extremity and right lower extremity numbness, as well as nausea with non-bloody emesis. While you are speaking to her, the pain migrates to her abdomen. On further history, she says she’s healthy and had a hysterectomy earlier this year. She has been told she has a heart murmur. She does not smoke or use drugs. Her father has a history of an aortic aneurysm that has not required surgical repair. Her vitals are: Temp, 97.9 F; Pulse, 101; BP, 94/51; Respiratory, 24; SpO2, 100%. On exam, she appears very distressed, pale, diaphoretic, and is vomiting clear emesis into a basin. Cardiovascular exam reveals a grade III diastolic decrescendo murmur and intact distal pulses. She is neurologically intact. Her EKG shows sinus tachycardia without acute ischemic changes. Pertinent labs include a normal chemistry panel, normal hemoglobin and hematocrit, negative troponin, and d-dimer >6000 ng/mL. You obtain a STAT CT angiogram of her aorta.


Pathophysiology
While aortic dissection is relatively rare, it remains the most common and lethal form of the acute aortic syndromes.1 The incidence is reported to range from 2.6 to 3.5 per 100,000 person-years.2 The inciting event for a classic aortic dissection involves a tear in the intimal layer, followed by blood entering the medial layer, forming a “false lumen.” From there, blood can propagate anterograde or retrograde, with varying catastrophic sequelae. Variants of aortic dissection include aortic intramural hematoma, intimal tear without hematoma, and penetrating atherosclerotic ulcer.2 Mortality rates remain as high as 30%, with immediate mortality rate up to 1% per hour after symptom onset.1,2
Aortic dissection has been classified according to the Stanford and DeBakey systems. Stanford is more widely used; it classifies dissections into those that involve the ascending aorta as type A, and all others distal to the left subclavian artery as type B.1,3 The DeBakey system classifies dissection based on the site of origin and is divided into types I, II, IIIa, and IIIb.1,3
Figure 3. Classification of aortic dissection. (Reproduced with permission from Manning WJ, Black JH. Overview of acute aortic syndromes. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on Jan. 23, 2018.) Copyright © 2018 UpToDate, Inc. For more information visit www.uptodate.com.)
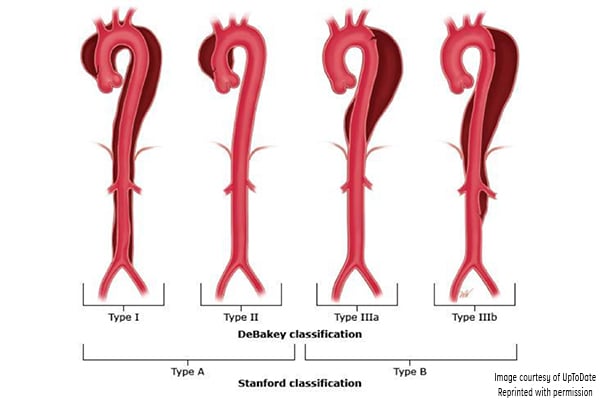
DeBakey classification
- Type I involves the ascending aorta, arch, and descending thoracic aorta and may progress to involve the abdominal aorta.
- Type II involves the ascending aorta.
- Type IIIa involves the descending thoracic aorta distal to the left subclavian artery and proximal to the celiac artery.
- Type IIIb dissection involves the thoracic and abdominal aorta distal to the left subclavian artery.
Stanford classification
- Type A involves the ascending aorta and may progress to involve the arch and thoracoabdominal aorta.
- Type B involves the descending thoracic or thoracoabdominal aorta distal to the left subclavian artery without involvement of ascending aorta.
Presentation
The most common symptom is acute pain, reported by approximately 85% of patients.3 Type A dissections present more often with anterior chest pain, while more Type B dissections present with back or abdominal pain, though overlap exists.3 The International Registry of Aortic Dissection says patients describe pain as sharp rather than ripping or tearing.3
Hypertension is present in Type B dissections (71%) more than Type A (36%).1,4 Conversely, hypotension, syncope, and shock are more common in Type A dissections. Hypotension and shock accompanying acute aortic dissection can result from cardiac tamponade, aortic hemorrhage, acute MI, true lumen compression by false lumen, aortic rupture, severe aortic regurgitation resulting in heart failure, or intra-abdominal catastrophe.1,4
Peripheral pulse deficits are present in 15-31% of patients, more commonly in Type A dissections.3,4 The classic “decrescendo diastolic murmur” of aortic regurgitation is found in approximately 40-50% of patients with Type A aortic dissection.3,5
It is estimated up to 40% of patients with aortic dissection present with acute neurologic findings, whether transient or permanent.6 This can mimic a stroke, especially in patients who present without pain (up to 15%).6 Neurologic manifestations can include altered mental status, TIA, stroke, encephalopathy, Horner’s syndrome, seizure, spinal cord ischemia, ischemic neuropathy or plexopathy, and nerve compression syndrome.6
Risk Factors
Risk factors for aortic dissection include conditions or mechanisms that result in weakening or degeneration of the aorta’s media layer, or place extreme stress on the aortic wall.4,5 Hypertension is the most important predisposing factor.2 Conditions that may transiently increase blood pressure, such as cocaine use, energy drinks, pheochromocytoma, and high-intensity weightlifting have also been associated with aortic dissection.1,2 Advancing age and male sex are also important risk factors. According to the IRAD, the mean age of patients studied was 63 years, and approximately two- thirds of patients were male.3,4
Major inherited connective tissue disorders known to be associated with aortic dissection include Marfan’s syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome.4,5 Turner’s syndrome, familial thoracic aortic aneurysm and dissection syndrome, and annuloaortic ectasia are also important genetic conditions associated with increased risk.4,5
Structural cardiovascular abnormalities associated with dissection include bicuspid aortic valve and coarctation of the aorta. Bicuspid aortic valve is one of the most common types of congenital heart disease.7 This variant predisposes the ascending aorta to aberrant, turbulent blood flow, and is often associated with underlying cystic medial degeneration.7 Dissection occurs 5-10 times more commonly in patients with bicuspid valve compared to tricuspid valve, and affects a younger population.7
Other risk factors include inflamma. tory conditions that cause vasculitis, pregnancy and delivery, trauma, and iatrogenic factors.2,5
Diagnosis
In addition to checking for pulse deficits, BP variations in the upper extremities (>20 mmHg), and a wide pulse pressure, an EKG and portable chest x-ray are quick bedside tests to evaluate for aortic dissection. According to the IRAD, EKG shows non-specific ST-segment or T-wave changes in 41%, no abnormalities in 31%, and ischemic changes in 15%.3 The classic widened mediastinum (measuring >8 cm at the level of the aortic knob), was present in 63% of Type A dissections and 56% of Type B dissections.2,3
D-dimer levels have been suggested as a “rule-out” marker. However, given that it is elevated in other acute chest conditions in the absence of dissection, it should be used to risk stratify patients when suspicion for the disease is low and reliance on the strong negative predictive value of the test is desired. In the IRAD study, d-dimer had a sensitivity of 97% and specificity of 47% in aortic dissection when using the cutoff of 500 ng/mL.8 The negative predictive value was 95% when used in the first 24 hours of symptom onset.8
CT angiography is the gold standard and diagnostic modality of choice.2 Consider transesophageal echocardio.graphy in the hemodynamically unstable patient, with the disadvantage of requiring esophageal intubation and procedural sedation.2 MR angiography can be used for stable patients but is not ideal in a crisis.2
Treatment
Initial management aims to stabilize the patient. When surgical management is indicated, notify the surgeon immediately. Minimize or avoid IV fluids in the hypoten.sive patient. Perform bedside cardiac ultrasound to evaluate for pericardial tamponade, valvular, or left-ventricular dysfunction.9 Hemodynamic instability, altered mental status, or airway compromise are indications for rapid-sequence intubation. An analgesic such as fentanyl (3 mcg/kg) should be given prior to induction to prevent the catecholamine release associated with intubation. Etomidate (0.3mg/kg) should be used as an induction agent for the patient who has known cardiovascular disease or is hemodynamically unstable.
Alternatively, in the hypertensive patient, use propofol (1.5 to 3 mg/kg), which suppresses catecholamine release (thereby reducing MAP). Use an arterial line to facilitate blood pressure management. Medical therapy is aimed at pain control and anti-impulse therapy. Morphine, hydromorphone, or fentanyl are acceptable analgesic agents. Anti-impulse therapy reduces left-ventricular contraction, thereby minimizing shear stress and propagation of the tear.9 The preferred antihypertensive agents are beta-blockers. Esmolol has a short half-life and is easily titrated, making it a good choice. The bolus dose is 250-500 mcg, followed by 25-50 mcg/kg/ minute. Labetalol is another option, given in sequential boluses or as a drip. An initial bolus can be given of 20 mg, followed by 40-80 mg every 10 minutes up to 300 mg total as needed. The dose for labetalol drip is 0.5-2 mg/minute. The target heart rate is <60 bpm, and systolic BP of 100-120 mmHg. If the target BP cannot be achieved, or there is a contraindication to beta-blockers, nitroprusside can be used — but heart rate must be controlled first, as it can activate the reflex sympathetic pathway.9
Acute aortic dissection involving the ascending aorta is a surgical emergency. Repair involves excising the intimal tear, obliterating the entry into the false lumen, using a synthetic interposition graft, and repair or replacement of the aortic valve.10 Surgery aims to halt progression of disease, improve flow, prevent rupture, and improve valve function.11
Uncomplicated Type B dissections are managed medically. Given that most patients with Type B dissections are hypertensive, medical management is aimed at BP reduction, typically with beta-blockers.1 Ongoing management for all aortic dissections focuses on anti-impulse therapy and serial imaging.10 Screening for associated genetic conditions is also important.10
Case Resolution
CT angiogram of the aorta revealed an extensive dissection of the non-dilated aorta, extending from the aortic root to the distal abdominal aortic bifurcation, into the bilateral common carotid arteries, as well as the bilateral proximal iliac arteries. This is consistent with a Type A dissection, a surgical emergency. Permissive hypotension was recommended to minimize expansion; she was given a 500cc NS fluid bolus, hydromorphone for pain, and antiemetics. During emergency surgery, she was found to have a bicuspid aortic valve. Surgery involved repair of the dissection with an ascending aortic supracoronary graft, suspension of the bicuspid aortic valve, and repair of the aortic root under deep hypothermic circulatory arrest using retrograde cerebral venous perfusion. Aortic valve replacement was not performed. She was discharged home 8 days later.
This case is notable because aortic dissection is rare in young, seemingly healthy, non-pregnant women, but underlying predisposing conditions (in this case, a bicuspid aortic valve) can be luring.
References
1. Braverman AC. Acute aortic dissection, clinician update. Circulation. 2010;122:184-188.
2. Manning WJ, Black JH. Clinical features and diagnosis of acute aortic dissection. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Accessed Jan. 23, 2018.
3. Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD), new insights into an old disease. JAMA. 2000;283(7):897-903.
4. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e296-e315.
5. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management, Part I: from etiology to diagnostic strategies. Circulation. 2003;108:628-635.
6. Gaul C, Dietrich W, Erbguth FJ. Neurological symptoms in aortic dissection: a challenge for neurologists. Cerebrovascular Disease. 2008;26:1-8.
7. Braverman AC. Clinical manifestations and diagnosis of bicuspid aortic valve in adults. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Accessed Jan. 13, 2018.
8. Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by d-dimer. Circulation. 2009;119:2702-2707.
9. Black JH, Manning WJ. Overview of acute aortic syndromes. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Accessed March 4, 2018.
10. Black JH, Manning WJ. Management of acute aortic dissection. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Accessed Jan. 23, 2018.
11. Stevens LM, Madsen JC, Isselbacher EM, et al. Surgical management and long-term outcomes for acute ascending aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1349-57.



