The Case.
A 43-year-old man presented to the ED with a 3-week history of a rash in a C7 dermatomal pattern that was burning and painful in nature. The rash began 3 weeks prior to the ED visit as a “group of little blisters” anatomically localized to the right upper back extending to the right arm. He mentioned having the shingles vaccine in the past. The patient was worried and in mild distress as the rash continued to burn, and he reported numbness to the area.
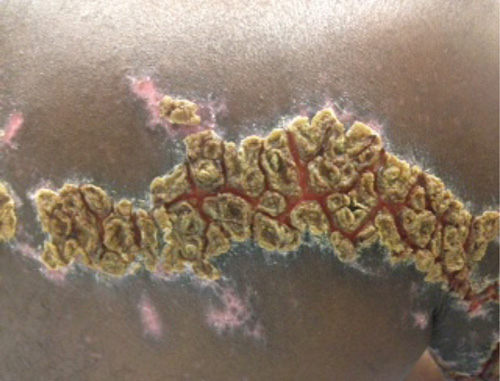
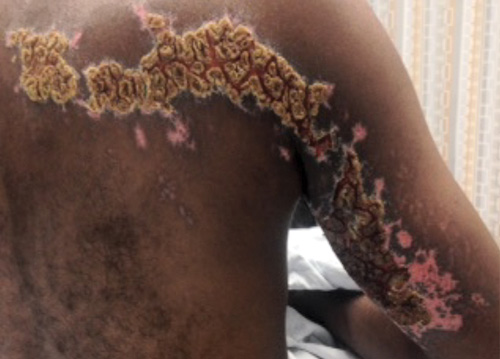
On physical examination, the rash was yellow, crusty, and tender upon palpation. The area was hyperkeratotic with noticeable scabbed lesions and cracked skin. The patient had normal range of motion without motor deficits in the right arm. The patient was discharged with tramadol, acyclovir ointment, clindamycin, and oral acyclovir. He was instructed to follow up with his primary care physician in 1-2 days.
What is the diagnosis?
Herpes zoster (HZ) is caused by reactivation of VZV, which occurs when immunity to VZV declines because of aging and/or immunosuppression. This condition most commonly affects the elderly population (more than half of the cases of HZ occur in patients >60 years).7 The condition is diagnosed clinically by the distinctive dermatomal characteristics of the rash and painful sensation. Herpes zoster lesions contain high concentration of VZV, which can spread through physical contact or airborne transmission. Exposure to airborne VZV can result in primary varicella infection in susceptible persons. However, HZ is only contagious from the time after the rash appears until the lesions crust.
The condition, commonly referred as shingles, comes from the Latin word cingulum meaning “belt,” which describes the rash’s dermatomal pattern. The rash is characterized as grouped vesicles on a red base that are located unilaterally (do not cross the midline) and undergo different stages: red macule to papule within the first 7-10 days progressing to
vesicles and ultimately pustules and crusty lesions. These crusty lesions may take up to 4 weeks or more to completely resolve. Herpes zoster presents with pruritus, dysesthesia, and pain along the involved dermatome. This pain may occur days before the onset of the rash, leading to multiple workups and misdiagnoses. Early detection and treatment of HZ reduces the acute symptoms and the severity of post-herpetic neuralgia, PHN.
Differential diagnoses for HZ include HSV, candidiasis, contact dermatitis, impetigo, autoimmune blistering diseases, and dermatitis herpetiformis. The most sensitive and specific diagnostic test to confirm the diagnosis of HZ is viral DNA PCR. However, PCR is not necessary if the clinical presentation is distinctive for HZ.
Herpes zoster (HZ) is caused by reactivation of VZV, which occurs when immunity to VZV declines because of aging and/or immunosuppression.
Complications
Common complications include post-herpetic neuralgia (PHN), superinfection with Staphyloccocus aureus and Streptococcus pyogenes, scarring, and hyperpigmentation. Shingles can also lead to pneumonia, hearing problems, blindness (herpes zoster ophthalmicus), encephalitis, cranial and peripheral nerve palsies, and sometimes death.
Approximately 13% of people 60-years-old and older with HZ will get PHN7, which mainly consists of pain persisting for more than 3 months after the initial shingles rash has healed. The risk of PHN increases with age and low immunity. In a large population study, the rate of PHN increased from 5% in HZ patients under 60-years-old to 10% in those ages 60-69 years and to 20% in those ages 80 years or older.2 The pain occurs due to viral damage to the sensory nerves. Since the condition is refractory to most analgesics, effective therapy often requires multiple drugs started in a systematic pattern, initially administering a low dose and increasing the dose gradually until the pain is resolved and/or adverse effects are noted. The medications used for PHN include: topical agents, antidepressants, anticonvulsants, and opioids.
Treatment
During an acute episode of HZ, symptom control and disease complication prevention is achieved with a combination of antiviral therapy, corticosteroids, and analgesics. The antivirals include acyclovir, famciclovir and valacyclovir, which inhibit viral replication and thus decrease the duration of viral shedding. This decrease allows for faster rash healing and reduces the duration of severe pain and the risk for PHN. Antiviral treatment is specifically recommended for patients older than 50 years who have moderate to severe pain and have facial involvement.3 Clinical trials demonstrate the effectiveness of treatment 72 hours after rash onset;3 however, in a clinical practice setting, this timing is not always feasible. Initiating antiviral treatment after 72 hours of rash onset and its benefits have not been studied, however, patients with severe HZ symptoms should be started on antivirals regardless of timing, especially if there is new vesicle formation. Valacyclovir and famiclovir may be administered in an outpatient setting because they require less frequent dosing than acyclovir. If a patient is immunocompromised, he or she requires a more aggressive, IV treatment. Corticosteroids do not have any effect on PHN; their only benefit is assistance in a faster resolution of the acute symptoms when combined with antiviral therapy. NSAIDs are ineffective in treating acute HZ pain; instead, acute pain can be relieved by antivirals, and associated HZ pain can be treated with opioids.
Vaccination Recommendations
The shingles vaccine, Zostavax, is a live attenuated vaccine given as a one-time, subcutaneous injection. It is composed of an antigen glycoprotein E (IgE) and an adjuvant system. Zostavax is the only shingles vaccine approved in the USA since 2006. The study by Cunningham et al. in 2016 found that the herpes zoster subunit vaccine reduces the risks of herpes zoster by 89.8% and post herpetic neuralgia by 88.8% among adults 70 years or older.3 The CDC recommends that people 60 years of age and older receive the shingles vaccine to prevent shingles and PHN.1 The 2015 Shingles Prevention Study gathered individuals ages 60 years and older who received the shingles vaccine and determined that the vaccine significantly reduced the incidence of shingles. The shingles vaccine decreases the risk of contracting shingles and PHN by 51% and 67% respectively.1 While these results suggest a benefit associated with early vaccination, there is still a risk for reactivation of VZV resulting in shingles as demonstrated by a study completed by Morrisson et al.4
One of the causes for shingles infection in vaccinated adults is the issue of decreased vaccination efficacy over time.
The Morrisson et al. study highlights the decline of the vaccine’s efficacy from years 7 to 11 post vaccination (46% decline in efficacy year 7 to 14% decline in efficacy in year 10).4 The downward trend of the vaccine’s efficacy throughout the years may be due to decreasing levels of vaccine-induced immunity and/or a decline in the host’s immunosenescence (immune response).
Although HZ vaccination is recommended for the elderly population as a preventative measure against shingles, research demonstrates the possibility of shingles infection despite being vaccinated. Current guidelines do not support revaccination with Zostavax; however, future studies may allow researchers to assess long-term protection of the HZ vaccine as well as the possible benefit of revaccination.
References
- CDC: What Everyone Should Know about Shingles Vaccine. https://www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed July 5, 2017.
- Yawn BP, Saddier P, Wollan P, St Sauver JL, Kurkland MJ, Sy LS. A population based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341-1349..
- Sampathkumar P, Drage LA, Martin DP (2009). Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274-80.
- Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. (2015) Clin Infect Dis. 2015;60(6):900-909.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR. Update on recommendations for use of herpes zoster vaccine. Morb Mortal Wkly Rep. 2014;63(33):729-31.



