Although asphyxiant exposure is a relatively uncommon phenomenon, it is important to keep in mind when evaluating an altered patient or a patient who has been found down, particularly in industrial or fire-related incidents.
Asphyxiants are dangerous substances that deprive the body of oxygen. They are separated into two categories, simple and systemic asphyxiants, based on their mechanism of action (Figure 1).
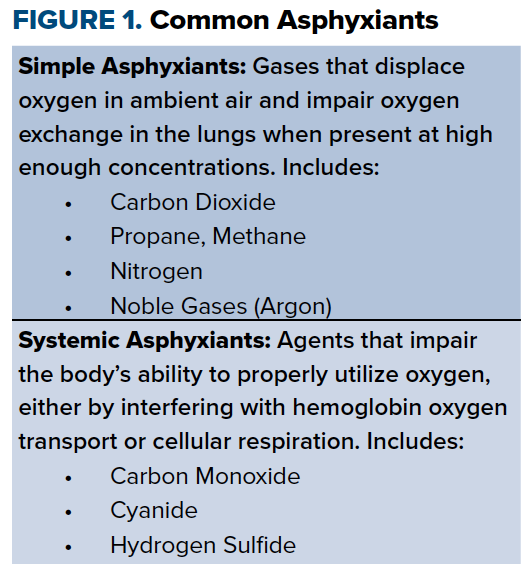
Simple asphyxiants include any gas that dilutes oxygen in ambient air and decreases the fraction of inspired oxygen (FiO2) below 21%.1 Examples include carbon dioxide, nitrogen, fuels like propane and methane, and noble gases like argon.
Systemic asphyxiants, on the other hand, work by interfering with oxygen transport or with intracellular utilization of oxygen. These include carbon monoxide (CO), cyanide (CN), and hydrogen sulfide (HS). Unlike simple asphyxiants, systemic asphyxiants are not limited to gases, and include nongaseous substances that can be ingested.2 If untreated, asphyxiant exposure can lead to myocardial damage, permanent neurological deficits, and death.
The most common source of asphyxiant poisoning is CO, with more than 50,000 cases annually in the United States primarily related to fires, motor exhaust, and gas leak.3 CN has been associated with occupational exposure in mining and manufacturing, and up to 35% of fire-related inhalational injuries are linked to CN toxicity.4,5 HS is a byproduct formed by the decay of organic material and the exposure risk is usually occupational, particularly for those working in oil drilling or with manure.1,6,7 Exposure to non-CO simple asphyxiants is less common, with the American Association of Poison Control Centers reporting about 2,500 cases in 2016. These are mostly related to industrial exposure while working in confined spaces.2, 8
General Approach to Management
When encountering a patient who potentially suffered asphyxiant poisoning, several steps in management should be taken regardless of exposure. First, a good history is essential in directing treatment. These patients may be found unconscious, and if brought in by ambulance, questions to ask EMS should include the circumstances in which the patient was found. If they were brought in after a fire, found in a confined space, or found in any industrial-type setting, asphyxiant toxicity should be included in the differential. For conscious patients, it can be helpful to ask if they recall any distinct odors prior to symptom onset. Many odorless simple asphyxiants such as propane are used as fuels and are injected with a substance with a "rotten egg scent" during manufacturing. HS has also been said to have this smell, whereas CN has been described to have a "bitter burning taste."2,9
History should focus on affected organ systems, including the nervous, cardiac, and respiratory systems. Neurologic presentations vary, ranging from headache and dizziness to seizure and coma. Likewise, cardiorespiratory symptoms including chest pain, dysrhythmias, shortness of breath, cough, and respiratory distress may be present.10 It is especially important to include asphyxiant exposure on your differential diagnosis when patients present with flu-like symptoms in the winter. During this time of year the flu is common, but so is CO exposure from patients bringing sources of heat such as stoves or generators indoors, and both diagnoses present with similar symptoms.
Combining physical exam with pertinent history findings is critical in guiding physicians towards the diagnosis of asphyxiant exposure (Figure 2).
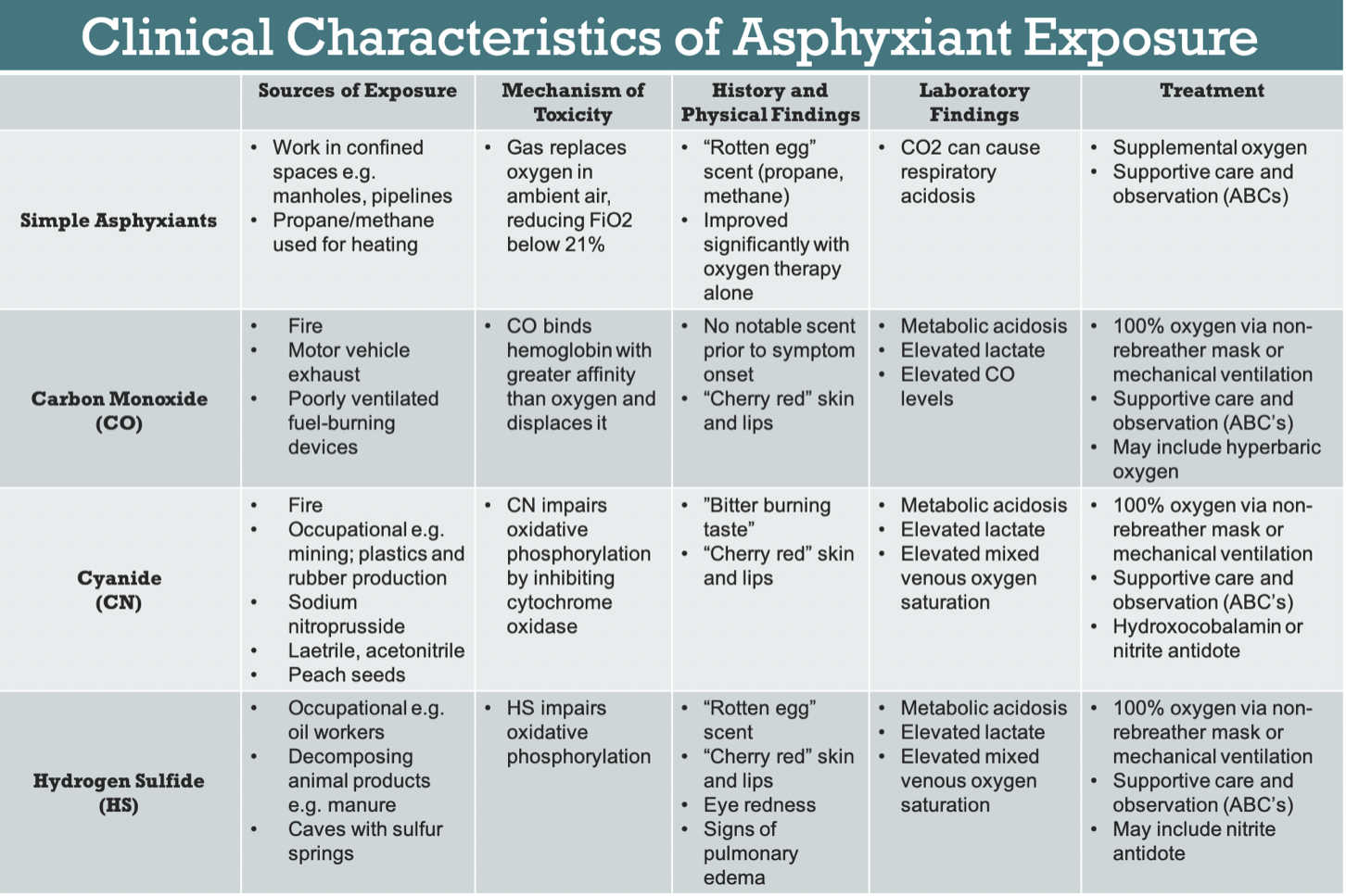 Nonspecific physical exam findings include altered mental status, respiratory distress, bradycardia, hypotension, and cardiac arrhythmia. Some classic signs include "cherry red" appearance of the skin and lips in CN and CO poisoning.3,10 Additionally, some asphyxiants like HS may also act as mucous membrane irritants, causing eye redness or signs of pulmonary edema.2 If a patient quickly and significantly improved with oxygen therapy alone, they are more likely to be suffering from simple asphyxiant exposure rather than systemic asphyxiants, the latter of which act in more complicated mechanisms than decreasing FiO2.1
Nonspecific physical exam findings include altered mental status, respiratory distress, bradycardia, hypotension, and cardiac arrhythmia. Some classic signs include "cherry red" appearance of the skin and lips in CN and CO poisoning.3,10 Additionally, some asphyxiants like HS may also act as mucous membrane irritants, causing eye redness or signs of pulmonary edema.2 If a patient quickly and significantly improved with oxygen therapy alone, they are more likely to be suffering from simple asphyxiant exposure rather than systemic asphyxiants, the latter of which act in more complicated mechanisms than decreasing FiO2.1
All patients treated for asphyxiant injury must be given supplemental oxygen and must be evaluated in a systematic manner. Assess major organ systems by beginning with the ABCs of evaluating Airway, Breathing, and Circulation. Once ABCs are secured, general management of systemic asphyxiant exposure must also include consultation of a poison control center and decontamination via removal of the clothes and lowpressure water irrigation.10
Systemic asphyxiant patients should receive 100% oxygen via non-rebreather mask or mechanical ventilation, as well as cardiac monitoring and serial EKGs due to a risk of myocardial damage and dysrhythmia. Other relevant diagnostics include arterial blood gas, serum lactate, and co-oximetry. Imaging should be guided by clinical exam and history, but may include a chest X-ray, as well as advanced brain imaging.2,9
Simple Asphyxiants
For simple asphyxiants, treatment focuses on removal from the causative agent, securing the ABCs, and providing supplemental oxygen. Patients suffering from simple asphyxiant exposure often receive oxygen therapy prior to arrival and can be significantly improved by the time they arrive to the ED. The patient should still be closely observed and monitored for several hours, depending on the exposure, and after discharge should be referred to outpatient followup for any potential delayed neurologic sequelae.1,2,11
Systemic Asphyxiants
Carbon Monoxide
Carbon monoxide works by binding hemoglobin with higher affinity than oxygen, significantly impairing oxygen carrying capacity and limiting its delivery to the tissues.3 Clinical management focuses on delivery of 100% oxygen via non-rebreather mask or mechanical ventilation. Untreated, carboxyhemoglobin has a half-life of about 4 to 5.5 hours.18 Importantly, in CO poisoning, oxygen saturation via standard pulse oximetry will appear normal despite hypoxia because this oxygen measurement method cannot distinguish between carboxyhemoglobin and oxyhemoglobin. Co-oximetry is required to do so, with laboratory methods being recommended over pulse co-oximetry.2 Nonsmokers have carboxyhemoglobin levels of about 3% or less, whereas smokers can have carboxyhemoglobin levels up to 10-15%.
Highflow oxygen can reduce the half-life of CO to 90 minutes and should be administered until the carboxyhemoglobin level is less than 5%.18 Additional care will focus on managing ABCs and potential complications, including metabolic acidosis, cardiovascular or neurological damage, and shock.1,2,3
Another treatment considered for CO poisoning is hyperbaric oxygen therapy (HBOT), which reduces the half-life of carboxyhemoglobin. The use of HBOT has been shown to decrease long-term neurologic dysfunction, but its use is not currently universally recommended for CO toxicity or for systemic asphyxiant exposure.3 If available, HBOT is generally accepted in patients with coma, signs of myocardial ischemia, neurological deficits, or a CO level greater than 25% in nonpregnant patients or greater than 15% in pregnant patients.3,12,13
Cyanide
Cyanide acts by impairing cytochrome oxidase of the mitochondrial electron transport chain and thereby inhibiting aerobic respiration.1 It is important to note that there are many cyanogenic agents including laetrile, acetonitrile, and even peach seeds that can be ingested and metabolized to CN, causing a delayed presentation.2 Because no rapid laboratory confirmatory test exists for CN poisoning, empiric treatment will likely be required. In addition to the history and physical discussed above, findings that can aid this diagnosis include severe lactic acidosis greater than 10 mmol/L, anion gap metabolic acidosis, elevated mixed venous oxygen saturation, and a normal pulse oximeter reading.10
Two antidotes are available for CN poisoning in the United States, the Cyanide Antidote Kit (CAK) and hydroxocobalamin. Numerous studies have demonstrated the efficacy and safety of hydroxocobalamin relative to CAK, and it has been recommended as the antidote of choice if available.10,14,15,16 The standard dose is 5g IV over 15 minutes; if the patient remains in critical condition, a second 5g IV dose can be given over 15 minutes to 2 hours.9 The CAK contains amyl nitrite, sodium nitrite, and sodium thiosulfate. Amyl nitrite perles are broken and given via inhalation for 30 seconds of every minute until an IV is established. Then, 300 mg of the sodium nitrite is infused over no less than 5 minutes. Finally, 12.5 g sodium thiosulfate is given IV over 10-20 minutes. If the patient remains in critical condition 30 minutes after the first dose, another half-dose can be given.1,2,9
A major drawback to the CAK is formation of a methemoglobin intermediate, which can be dangerous in smoke inhalation patients because they may have concurrent CO toxicity already reducing hemoglobin oxygen carrying capacity.2 Additionally, the components of the CAK can cause serious side effects including hypotension and psychosis.1,2
Hydrogen Sulfide
Hydrogen sulfide works as a systemic asphyxiant via inhibition of cellular respiration in a similar manner to CN.7 HS exposure does not benefit from the same body of treatment data as other asphyxiants and does not currently have any proven antidote. Nitrite therapy via the CAK has been recommended if it can be given shortly after exposure, following the same procedure above, excluding sodium thiosulfate.2,7,17
Conclusion
Although asphyxiant exposure is a relatively uncommon phenomenon, it is important to keep them in mind when evaluating an altered patient or a patient who has been found down, particularly in industrial or fire related incidents. It is also important to consider these diagnoses in patients who present with vague symptoms, as the history may be the key to narrowing in on the diagnosis. Exposure can leave people unconscious and unable to provide a history, and because of how rapidly these agents can kill, confirmatory testing is often unable to provide a definitive diagnosis in a clinically relevant time frame.
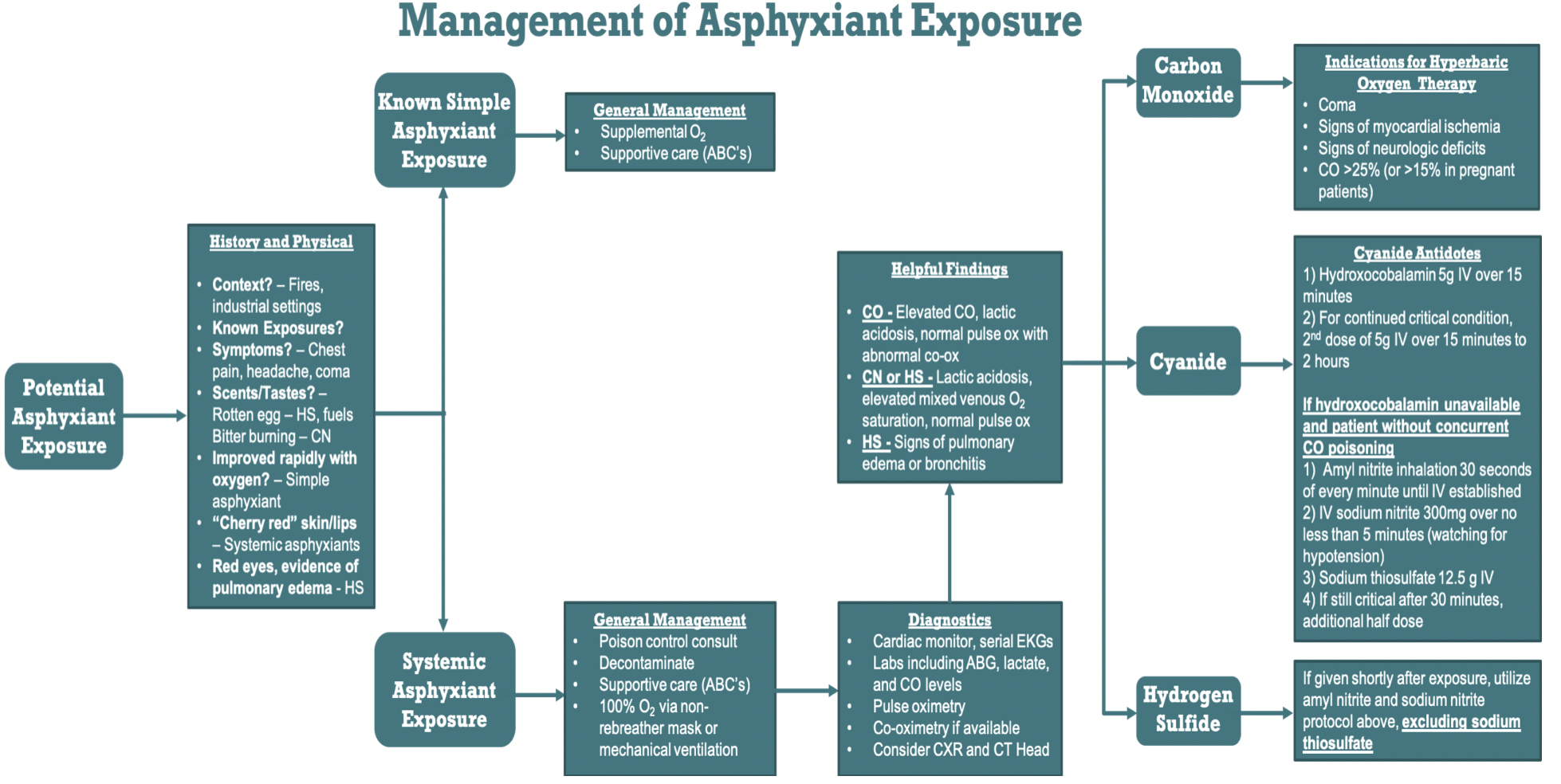
Providers must be ready to move quickly if asphyxiant poisoning is suspected and remembering the basics of presentation and treatment can save critical time in the management of these conditions (Figure 3).
References
1. Tan K, Wang T. Asphyxiants: Simple and Chemical. Ann Disaster Med. 2005;4 Suppl 1:S35-S40
2. Borron SW, Bebarta VS. Asphyxiants. Emerg Med Clin North Am. 2015;33(1):89-115.
3. Rose JJ, Wang L, Xu Q, et al. Carbon Monoxide Poisoning: Pathogenesis, Management and Future Directions of Therapy. Am J Respir Crit Care Med. 2016
4. Sauer SW, Keim ME. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann Emerg Med. 2001;37(6):635-41.
5. Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325(25):1761-6.
6. Tustin AW, Jones A, Lopez GP, Ketcham GR, Hodgson MJ. Occupational chemical exposures: a collaboration between the Georgia Poison Center and the Occupational Safety and Health Administration. Clin Toxicol (Phila). 2017;1-8.
7. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
8. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
9. Toxic Substances Portal - Cyanide. Centers for Disease Control and Prevention. https://www.atsdr.cdc.gov/mmg/mmg.asp?id=1073&tid=19. Published October 21, 2014. Accessed January 2, 2019.
10. Thomas A, Lipp C. CRACKCast E159 - Inhaled Toxins. CanadiEM. https://canadiem.org/crackcast-e159-inhaled-toxins/. Published March 1, 2018. Accessed December 5, 2018
11. Permentier K, Vercammen S, Soetaert S, Schellemans C. Carbon dioxide poisoning: a literature review of an often forgotten cause of intoxication in the emergency department. Int J Emerg Med. 2017;10(1):14.
12. Hampson NB, Dunford RG, Kramer CC, Norkool DM. Selection criteria utilized for hyperbaric oxygen treatment of carbon monoxide poisoning. J Emerg Med. 1995;13(2):227-31.
13. Kao LW, Nañagas KA. Carbon monoxide poisoning. Emerg Med Clin North Am. 2004;22(4):985-1018.
14. Dumestre D, Nickerson D. Use of cyanide antidotes in burn patients with suspected inhalation injuries in North America: a cross-sectional survey. J Burn Care Res. 2014;35(2):e112-7.
15. Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013;21:31.
16. Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49(6):794-801, 801.e1-
17. Toxic Substances Portal - Hydrogen Sulfide Carbonyl Sulfide. Centers for Disease Control and Prevention. https://www.atsdr.cdc.gov/mmg/mmg.asp?id=385&tid=67. Published October 21, 2014. Accessed January 2, 2019.
18. Peter CF, Manaker S, Perry H. Carbon monoxide poisoning. Traub SJ, Burns MM, Grayzel J, ed. UpToDate. Waltham, Mass: UpToDate 2019. Accessed 4/22/2019.